Fig. 2.1
Organization of the HPV16 viral genome showing the function of the viral genes (Diagram adapted by Heather Walline)
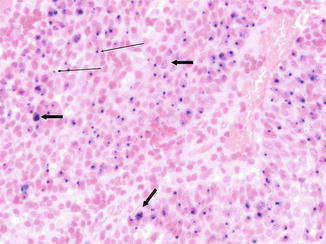
Fig. 2.2
HPV in situ hybridization of an HPV-induced oropharynx tumor (viral genome stained blue). Multiple episomes are present in many cells (thick arrows). Integrated viral DNA in cells lacking high copy number is consistent with a single signal in other cells (thin arrows). Note that normal stromal cells lack a viral signal (Photomicrograph by Jonathon McHugh)
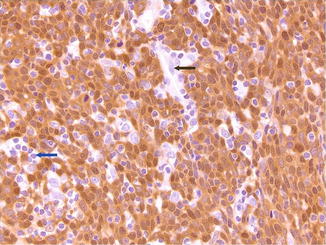
Fig. 2.3
Top: Strong nuclear and cytoplasmic p16 staining of a section from an HPV+ oropharynx tumor. Lymphocytes (blue arrow) and endothelial cells (black arrow) are unstained (Photomicrograph by Jonathon McHugh)
2.1.6 HPV in Head and Neck Cancer
In the late 1980s and early 1990s, our group and others began to search for high-risk papillomaviruses in head and neck cancers. This search was based on the observations that HPVs were a cause of genital mucosal tumors and that HPV6 and HPV11 were already known to be involved in vaginal condylomas and laryngeal papillomatosis [50, 51]. HPV types were also known to be associated with Schneiderian papillomas (inverting papillomas) of the nasal sinus [52]. It was postulated that if these relatively benign mucosal HPV types known to be associated with vaginal and cervical condylomas could also infect laryngeal and nasal mucosa, then it was reasonable that high-risk HPV types associated with carcinoma of the uterine cervix might also infect the mucosa of the upper aerodigestive tract. In 1996, Franceschi et al. [53] reviewed the evidence for HPV involvement in cancers of the upper aerodigestive tract. By this time there were numerous reports of HPV involvement in head and neck cancers, but due to tremendous variation from laboratory to laboratory, there was great uncertainty about the validity of those studies. Snijders et al. seemed to have the most consistent [54–58] and convincing data from high-risk HPV involvement in head and neck cancer sites in the early 1990s. With the use of generalized primers to amplify 11 different HPV types combined with sequencing [59] and the confirmation of expression of viral early region E6/E7 transcripts, it became clear that these findings were not just the result of PCR cross-contamination or experimental error. Snijders et al. reported finding tonsil tumors containing HPV16 (n = 2) and HPV33 (n = 2) expressing alternate E6–E7 splice variants (E6*I, E6*II, and E6*III), confirming viral oncogene activity in these tumors which was consistent with virally induced carcinomas [55, 57, 60]. By 1994 they reported finding HPV in 12/24 tonsil cancers (7 HPV16, 2 HPV33, 1 HPV7, 1 HPV16/33, and 1 HPV33/59); additionally they noted that p53 was sometimes expressed in HPV-positive cancers [61].
2.1.7 HPV-Related Head and Neck Cancers and Sexual Behaviors
Based on the evidence that genital HPV-related cancers were linked to sexual contacts, it was logical to examine the role of sexual transmission in oral HPV-related cancers. In 1998 Schwartz et al. [62] acknowledging the accumulating evidence for oncogenic HPVs as etiologic factors in oral squamous cell carcinomas (SCC) carried out a case-control study to determine the risk of cancer related to HPV infection and sexual practices. They found that for men, oral SCC risk increased with decreasing age at first intercourse, increasing number of sex partners, and a history of genital warts. They also found a link between HPV, smoking, and risk of oral cancer. This study supported sexual transmission of high-risk HPV as a risk for cancer development although detection of oral HPV was similar in cases and controls.
In 2000, Gillison et al. [63] presented evidence for a causal role for HPV in a relatively large subset of head and neck cancers. Of 253 patients with head and neck SCC (HNSCC) 62 (25 %) contained HPV, most (56/62) of these contained HPV16. Southern blot hybridization patterns were consistent with viral integration; the tumors with HPV were largely poorly differentiated and associated with oropharyngeal tumor site. When compared with HPV-negative oropharyngeal tumors, HPV-positive tumors were less likely to be from high alcohol and tobacco users and were less likely to have p53 mutations. Importantly after adjustment for age >60 and heavy alcohol consumption, patients with HPV-positive tumors were found to have a much lower risk of death from cancer when compared to those with HPV-negative tumors. In the decade from 2000 to 2010, it became well established that high-risk HPV has an important etiologic role in the development of oropharyngeal cancer [64–77]. In 2008, Gillison et al. [78] reported an analysis of the risk factors for HPV16-positive and HPV16-negative tumors among HNSCC cases diagnosed in the years 2000–2006. Two to one controls (without HNSCC, matched for age and sex) were compared to identify risk factors for HPV16+ cancer. Of the 240 cases, 94 had HPV16-positive tumors. HPV16-positive HNSCC was independently associated with sexual behaviors and exposure to marijuana, but not cumulative measures of tobacco smoking or alcohol drinking or poor oral hygiene. Associations increased with number of oral sex partners and increasing marijuana use. This contrasted with HPV16-negative cancers which did have strong associations with tobacco smoke, alcohol consumption, and poor oral hygiene. As in cervical cancer, this study pointed to sexual behaviors as a mechanism of HPV infection and elevated risk of HPV-induced cancer as well as highlighting the distinct etiology of two classes of HNSCC.
Although retrospective studies all indicated a better prognosis for HPV-related head and neck cancers, the proof came in a prospective ECOG trial reported by Fakhry et al. in 2008 [66]. Induction chemotherapy followed by concurrent chemoradiotherapy (RT) in patients with stage III and IV oropharynx cancer. Compared with patients with HPV-negative tumors, patients with HPV-positive tumors had higher response rates to both induction chemotherapy and chemoradiation and after a median follow-up of 39.1 months had improved overall survival (OS) (2-year OS = 95 % [95 % CI = 87–100 %] vs. 62 % [95 % CI = 495–74 %], with a difference of 33 % [95 % CI = 18.6–47.4 %]).
In our own studies, we assessed the rates of HPV infection in three different oropharynx groups from clinical studies spanning a 12-year period from 1999 to 2011. The proportion of HPV tumors was already high in the 1999–2001 cohort represented by UMCC-9921 [76].Of patients in this study with oropharynx cancers, tissue blocks were available from 41, of these only 14 were negative for HPV DNA. The difference in disease-specific survival between the patients with HPV-positive tumors (blue line) and those with HPV-negative tumors (red line) was dramatic in this group as shown in Fig. 2.4 [73, 76]. Further analysis of this cohort revealed that biomarkers, including HPV titer, p16, EGFR, and smoking all influenced response to induction chemotherapy and response to concurrent chemo-RT. High EGFR expression was directly correlated with current smoking and lower HPV titer in the tumor and inversely associated with disease-specific and overall survival, while low EGFR expression and high p16 (or high HPV titer) expression are associated with good response to therapy and outcome.
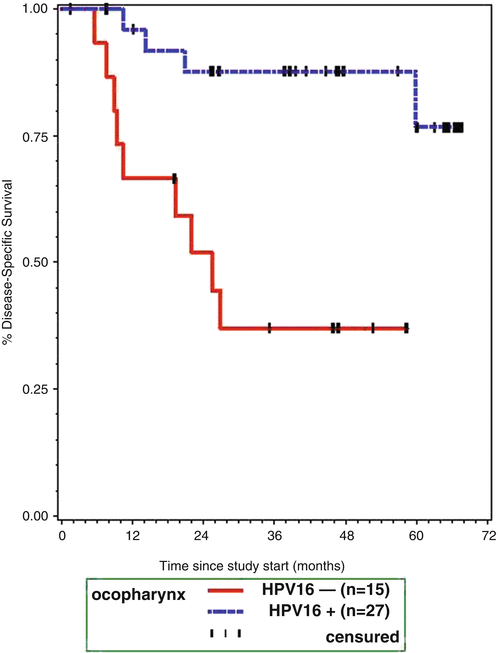

Fig. 2.4
Disease-specific survival in oropharynx cancer patients, by hrHPV status (Reprinted from Kumar et al. [89]. With permission)
In a subsequent cohort consisting of patients in the UMCC-0221 oropharynx cancer trial [79], enrolled from 2002 to 2005 and treated with intensity-modulated conformal radiation and concurrent cisplatin chemotherapy (100 mg/m2; given every 21 days) designed to reduce dysphagia, we found that 75/83 (90 %) tumors contained high-risk HPV [74]. The patients in this trial enjoyed 3-year disease-free survival of 88 % that reflects the high HPV-positive rate in this study group, the efficacy of the therapy, and the suitability of tumor location for the trial. In a third cohort treated with the same regimen as UMCC-0221 (but not part of a clinical trial) 71/77 (92 %), oropharynx tumors were positive for hrHPV. This finding is consistent with the increasing incidence of oropharynx cancer and the increasingly important role of HPV in these tumors.
Smoking status effects outcome in HPV-positive oropharynx SCC
Although smoking is much less common in HPV-positive than in HPV-negative oropharynx squamous cancer (OPSC) patients, we found that more than 60 % of the patients with oropharyngeal cancer in the UMCC-9921 and UMCC-0221 trials were current or former smokers. Of patients with HPV-positive OPSC, current and former smokers had a greater risk of recurrent or metastatic disease (Table 2.1). We also noted a trend for reduced disease-specific survival in current as compared to never smokers [74]. This observation was subsequently substantiated in a reassessment of the national RTOG trial.
Table 2.1
Risk of recurrence and disease-specific survival (DSS) by tobacco use among HPV-positive patients
|
Tobacco group
|
Hazard ratio
|
95 % C.I.
|
p value
|
|
|---|---|---|---|---|
|
Risk of recurrence
|
Current vs. never
|
5.2
|
(1.1–24.4)
|
0.038*
|
|
Former vs. never
|
2.9
|
(0.6–13.6)
|
0.18
|
|
|
Current vs. former
|
1.8
|
(0.7–4.8)
|
0.24
|
|
|
DSS
|
Current vs. never
|
7.2
|
(0.88–58.4)
|
0.07
|
|
Former vs. never
|
3.6
|
(0.43–30.1)
|
0.24
|
|
|
Current vs. former
|
2
|
(0.66–6.03)
|
0.22
|
Analysis of p16 overexpression as a surrogate for HPV involvement combined with retrospective PCR analysis of HPV DNA in the tumors, in the national RTOG 0129 clinical trial, confirmed that patients with HPV-positive tumors enjoyed better survival than those with HPV-negative tumors but also illustrated the adverse effect of smoking on outcome in this group [80]. Of 266 patients with OPSC, HPV status and pack year smoking history, as well as T-classification and N-classification allowed assignment of patients into low-, intermediate-, and high-risk categories for overall survival after treatment with concurrent cisplatin and radiation. Of the 266 patients in the trial, 178 patients had HPV-positive tumors and 88 had HPV-negative tumors. Of the patients with HPV+ tumors, 88 had smoking history of less than ten pack years and all fell into the low-risk group. The remaining 90 HPV-positive cases had more than ten pack years, 26 of those had N0–N2a nodal status and also fell into the low-risk group which enjoyed three-year overall survival rates of 93 % (95 % C.I. 88.3–97.7). For the other HPV-positive smokers (>10 pk/year), 64 had N2b–N3 nodal status and these fell into the intermediate-risk group – 3-year overall survival of 70.8 % (95 % C.I. 60.7–80.8). All 88 HPV-negative cases fell into either the intermediate-risk group n = 15 with <10 pk/year and T2–T3 tumors or the high-risk group which consisted of eight HPV-negative patients with <10 pk/year, but T4 tumors and 65 HPV-negative patients with >10pk/year who had a three-year OS rate of 46.2 % (95 % C.I. 34.7–57.7). This study again demonstrated that HPV status was associated with better OS but that tobacco smoking reduces the survival advantage of HPV-positive tumors.
2.1.8 Sexual Practices Are Linked to HPV-Positive Head and Neck Cancer
D’Souza et al. [81] carried out a case-control study published in 2007 comparing 100 oropharynx squamous cancer (OPSC) patients to 200 ear, nose, and throat patients without cancer. Their findings strongly linked sexual practices with oropharyngeal cancer. Affected individuals reported much higher numbers of lifetime vaginal sex partners (26 or more) (odds ratio, 3.1; 95 % confidence interval (CI), 1.5 to 6.5) and/or oral sex partners (6 or more) (odds ratio, 3.4; CL 1.3 to 8.8) which conferred threefold increased risk of oropharynx cancer. This study also showed significant association of oral infection by HPV16 (or any of 37 other HPV types) with the presence of HPV in the tumor and serologic evidence of antibodies to HPV oncoproteins and L1 capsid protein. Seropositivity was associated with history of tobacco and alcohol use and was also present in those without a strong tobacco and alcohol history. The association with OPSC was also increased in those with oral HPV infection, whereas heavy tobacco and alcohol use showed an increased association with OPSC in those without evidence of exposure to HPV16. This seminal paper was the convincing evidence that HPV was clearly a risk factor for oropharyngeal cancer and that transmission of the virus was strongly associated with sexual behavior.
The prevalence of HPV-positive tumors in the oropharynx is largely due to frequent origin of these tumors from the tonsils, either the palatine (faucial) or lingual tonsils. Cancers arising from the lingual tonsil often present as tumors of the base of tongue, due to the close proximity of the lingual tonsil to the base of tongue. Interestingly, Shiboski et al. [82] stimulated by reports of increasing cancers of the tongue and tonsil in young men in the USA and Europe, and the association between HPV infection and tonsil cancer evaluated the Surveillance, Epidemiology, and End Results (SEER) 1973–2001 database for site-specific incidence for tongue and tonsil cancer in young men (ages 20–44). This revealed that in this young male age group, whereas there was a decreasing incidence of cancer at oral cavity sites other than tongue, there was an annual 3.9 % change in the incidence of tonsil cancer and an annual 1.7 % change in the incidence of tongue cancer (Fig. 2.5). This pattern of change is consistent with the changing epidemiology of head and neck cancer.
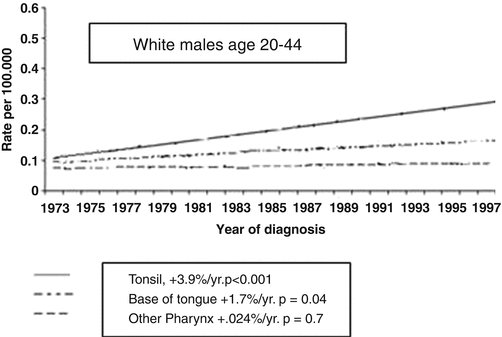

Fig. 2.5
Changing incidence of oropharyngeal cancer in the USA (Modified from Shiboski et al. [82]. With permission)
Smoking incidence has decreased dramatically over the past 30 years and cancer incidence for smoking-related head and neck cancers has also changed. At the same time the incidence of HPV-positive head and neck cancers has increased dramatically and an important part of the increase in these tumors has been in younger men [83].
The increase in the frequency of HPV-positive oropharynx cancer represents the changing epidemiology of head and neck cancer in the USA and Europe over the past 20 years. Tobacco use has declining significantly and its position as the most important cofactor in head and neck cancer is eroding, leading to a decrease in the incidence of laryngeal cancer, while at the same time the incidence of oropharyngeal cancer is increasing. Over the past 30 years, the incidence of cervical cancer has been declining. This is in large part due early detection and treatment. The Papanicolaou test or PAP smear takes swabs of cells from the uterine cervix and examines them for abnormal cells under the microscope. This test detects the early stages of cervical cancer, and with careful follow-up, hrHPV testing, and removal of early cancers, the incidence of invasive and lethal cervix cancer has fallen dramatically. In contrast, there is no early detection test for OPSC and most patients present with advanced stage 3 or 4 disease; furthermore OPSC incidence in the USA has increased to the point where the number of newly diagnosed oropharynx cancers surpassed the incidence of cervical cancer in 2013, with 12,989 cases of oropharynx cancer and 11,388 cases of cervical cancer (SEER Data 2013).
The predilection of HPV-positive tumors for the oropharynx is in part due to the tonsils as a key site of infection. Human papillomaviruses (HPV) must infect basal epithelial cells to establish a productive infection. In oropharyngeal squamous cell carcinoma (OPSCC), the crypt epithelium overlying the lymphoid tissue of the tonsils is a common site of HPV infection (Fig. 2.6). The epithelium overlying the tonsil tissue is thin and the tonsil is also a site of frequent inflammation due to infection with a variety or microorganisms. Inflammation can alter the integrity of the epithelium which can facilitate the virus having access to the basal cell layer. Once the virus gains access to and infects the basal cell layer, it induces replication of the infected cells and replication of the viral genome. As the cell differentiates, the capsids are produced, the viral DNA is packaged, and at the surface of the epithelium, infectious virus is released. Eventually the infection becomes latent and no infectious virus is produced, although HPV DNA may be detected in desquamated cells. Generally HPV infections persist for 18 months to 2 years. In rare cases malignant transformation occurs. The factors that lead to oral HPV infection and the development of cancer are becoming better known. Oral HPV infections are far more common in men than in women [84] and it appears that men often acquire the virus through oral sex [85]. Many women develop genital HPV infections soon after becoming sexually active. It is likely that women become immunized following genital infection, as indicated by the presence of antibodies to the viral proteins, whereas it seems that genital exposure in men may be less likely to lead to adequate immune resistance to prevent a subsequent mucosal infection. This provides one explanation for the 9:1 male to female ratio of HPV-positive oropharyngeal cancers.
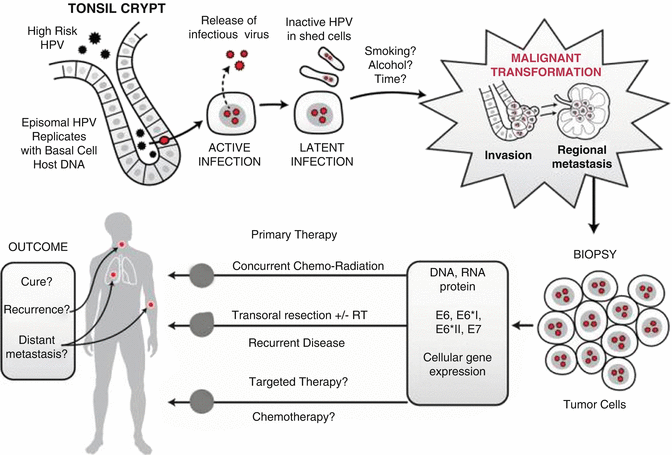

Fig. 2.6
HPV infection in tonsil tissue, malignant transformation, metastasis, therapy, and outcome
2.1.9 HPV in HNSCC at Sites Other Than the Oropharynx
The high frequency of HPV involvement in OPSC and the common finding of HPV16 have suggested that these are the only important players for HPV in HNSCC. However, as we were examining our HPV testing data for a large cohort of OPSC patients, we also tested some nasopharyngeal tumor specimens treated at our center. In looking through the data, we found that four of the five NPC tumors contained high-risk HPV and that the HPV types were more varied than typically observed in OPSC [86]. Nasopharyngeal cancer (NPC) has been etiologically linked to the Epstein-Barr virus (EBV), but many North American patients with NPC are negative for EBV in their tumors. Four of the five tumors reported by Maxwell et al. [86] were positive for p16 and negative for EBER (a test for active EBV) (Fig. 2.7). The four p16-positive tumors contained HPV18 in two, HPV16 in one, and HPV59 in one. Thus not only was HPV implicated in NPC in some patients, but the involved HPV types were heterogeneous. The HPV-negative NPC was from an Asian woman who had emigrated to the USA and her tumor was p16 negative, HPV negative and exhibited strong EBER staining, consistent with EBV as the etiologic agent. We carried out a follow-up study of 61 NPC archived tumors. In this set we found 26 (43 %) were EBV positive, 18 (30 %) were HPV positive, and 17 (28 %) were HPV negative for both viruses. Of the HPV-positive cases, 94 % were of white Midwestern backgrounds. In contrast to the OPSC, the survival was better among the EBV-positive cases than the HPV-positive cases, but both types of virus-positive tumors were associated with belter outcome than that of the patients with double-negative NPC [87]. In that study there was also the appearance of a trend for more frequent HPV-positive NPC tumors with time, suggesting that like tumors of the oropharynx, that sexual transmission of HPV to the lymphoid tissue of the nasopharynx was also increasing in frequency. In addition to nasopharynx cancers, we are also finding that HNSCC at other sites also contain HPV, but at lower frequency. In a survey of head and neck tumors in our head and neck SPORE biorepository, we found high-risk HPV types in about 10 % of 356 oral cancers and 11 % of 164 larynx cancers. We do not yet have enough information to determine if there is an increasing trend for HPV-positive tumors at these sites, but it would not be surprising to learn that the incidence of HPV-induced tumors at these sites is also increasing. It is also likely that the increase will be in younger patients who have lower tobacco use and more sexual partners [88].
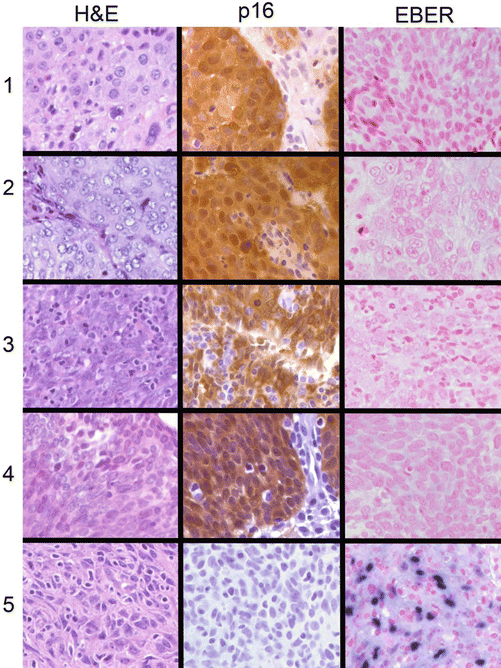

Fig. 2.7
Histology, p16 expression, and EBER expression in 5 nasopharyngeal tumors. Left column H&E-stained tumor; middle column p16 staining; right column EBER staining (Reproduced with permission from Maxwell et al. [86])
Whether widespread use of the high-risk HPV vaccines will also reduce the incidence of these tumors remains to be determined. Currently there is comparatively low penetrance of the vaccine in male adolescents in the USA. The HPV vaccines have shown a reduction in cervical HPV infection in young women; however, the impact of the vaccine on oropharynx cancer or even on oral HPV infection is still uncertain.
References
1.
2.
DiMaio D. Nuns, warts, viruses, and cancer. Yale J Biol Med. 2015;88(2):127–9.PubMedCentral
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


