http://evolve.elsevier.com/Haveles/pharmacology
The opioid analgesics are often used to manage dental pain in patients in whom nonsteroidal antiinflammatory drugs (NSAIDs) are contraindicated. The dental hygienist and the dentist should be aware of the opioid groups, side effects, relative potency, and proper place in the management of dental pain.
History
Opium is the dried juice from the unripe seed capsules of the opium poppy. As early as 4000 bc, many cultures had recognized the euphoric effect of the poppy plant. In the early 1800s, morphine and codeine were isolated from opium. Until about 1920, patent medicines (medicines whose efficacy and safety were questionable) containing opium were promoted for numerous uses. When these agents, used orally, became unlawful, narcotic (opioid) abuse by injection began and has continued until the present.
Classification
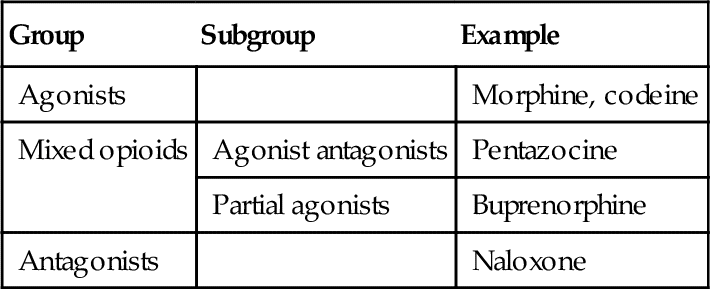
The clinically useful opioids may be divided in several different ways. One way is by their mechanism of action at the receptor sites: agonists, mixed opioids, and antagonists. Table 6-1 shows the classifications.
Mechanism of action
The opioids bind to receptors located in both the central nervous system (CNS) and the spinal cord, producing an altered perception of reaction to pain. Receptors that mediate specific pharmacologic effects and adverse reactions are stimulated to varying degrees by individual opioids.
The discovery of three groups of endogenous substances with opioid-like action, the enkephalins, endorphins, and dynorphins, has helped explain the presence of these receptors. These naturally occurring peptides possess analgesic action and have addiction potential. They probably function as neurotransmitters, but their exact function has not been elucidated. They may be involved in the analgesic action of a placebo and the enhancement of well-being that occurs with running (an increase in β-endorphins).
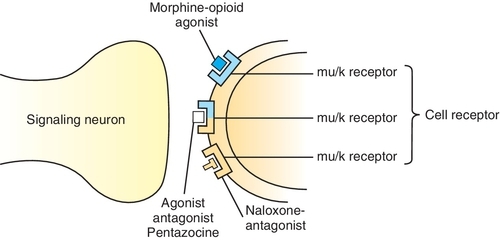
Table 6-2 describes the pharmacologic effects of selected opioid receptors and the effect of some opioids on these receptors. Opioids may be complete agonists, partial agonists, agonist-antagonists, or antagonists. The three opioid receptors that have been characterized in more detail and that are stimulated by the opioids are the mu (μ), kappa (κ), and delta (δ) receptors. Differences in affinity for and action of different opioids in tolerance to pain might even be the result of variations in the endogenous levels of the neurotransmitters. Differences in affinity for and action of different opioids at these and other specific receptors explain some of the distinctions among the different opioids’ adverse reactions. For example, stimulation of μ-receptors produces analgesia. The κ-receptor is responsible for dysphoria. Pentazocine, a κ-receptor agonist, produces dysphoria; morphine has little effect on the κ-receptor and causes less dysphoria than pentazocine. Naloxone is an antagonist at the three receptor sites (Figure 6-1). Other opioid receptors include sigma (σ) and epsilon (ε). The stimulation of σ-receptors is associated with autonomic stimulation, dysphoria, hallucinations, nightmares, and anxiety. It is thought that the stimulation of ε-receptors may result in analgesia. More opioid receptors are sure to be identified and characterized. As more subreceptor types are elucidated, it will be possible to further separate beneficial (analgesic) effects of the opioids from their side effects (e.g., respiratory depression, constipation, and drug dependence).
Table 6-2
Opioid Receptors, Effects, and Stimulation by Various Opioids
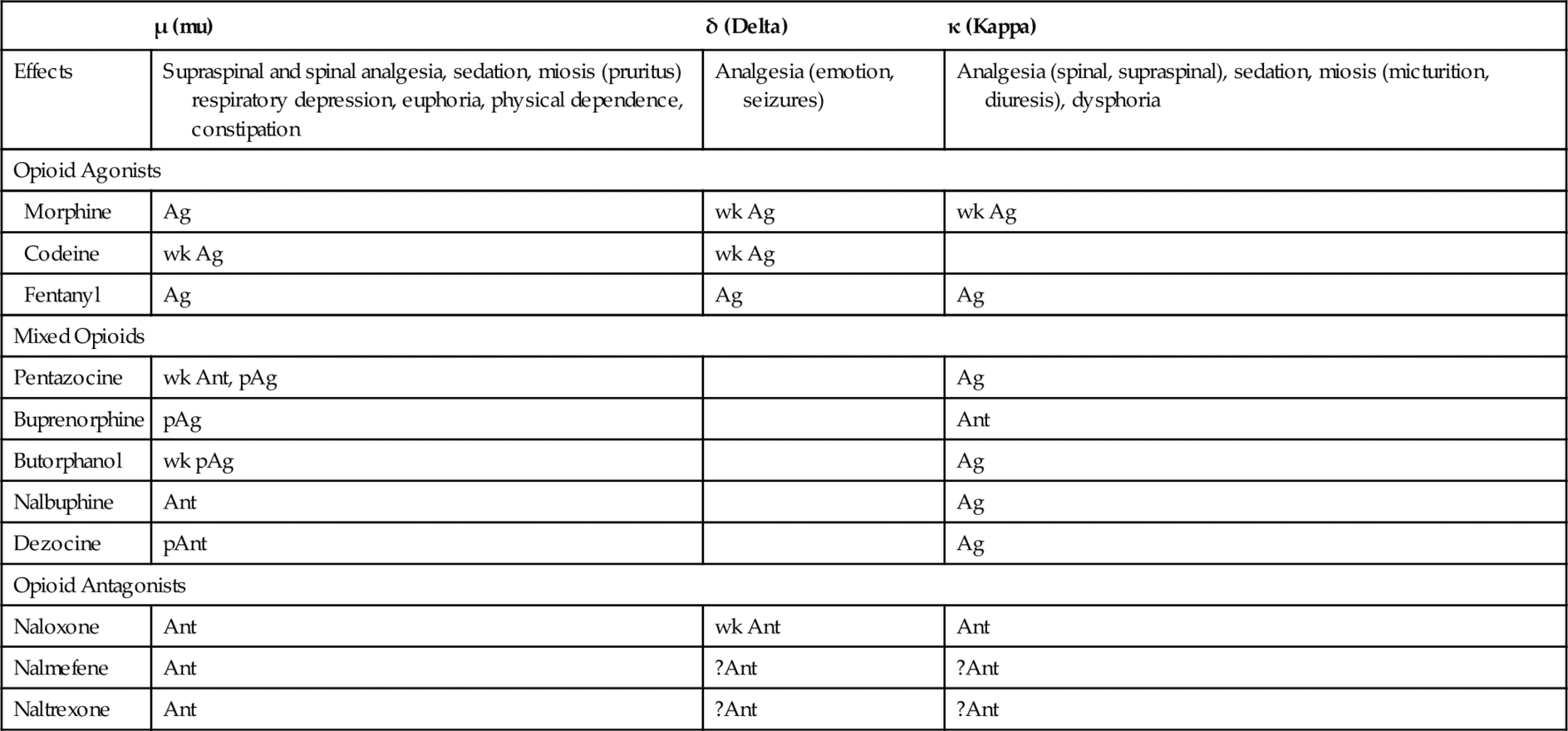
Ag, agonist; Ant, competitive antagonist; pAg, partial agonist; pAnt, partial antagonist; wk, weak; ?, unknown.
Pharmacokinetics
ADME, which is an acronym formed from the first letters of the components of drug handling, refers to absorption, distribution, metabolism, and excretion:
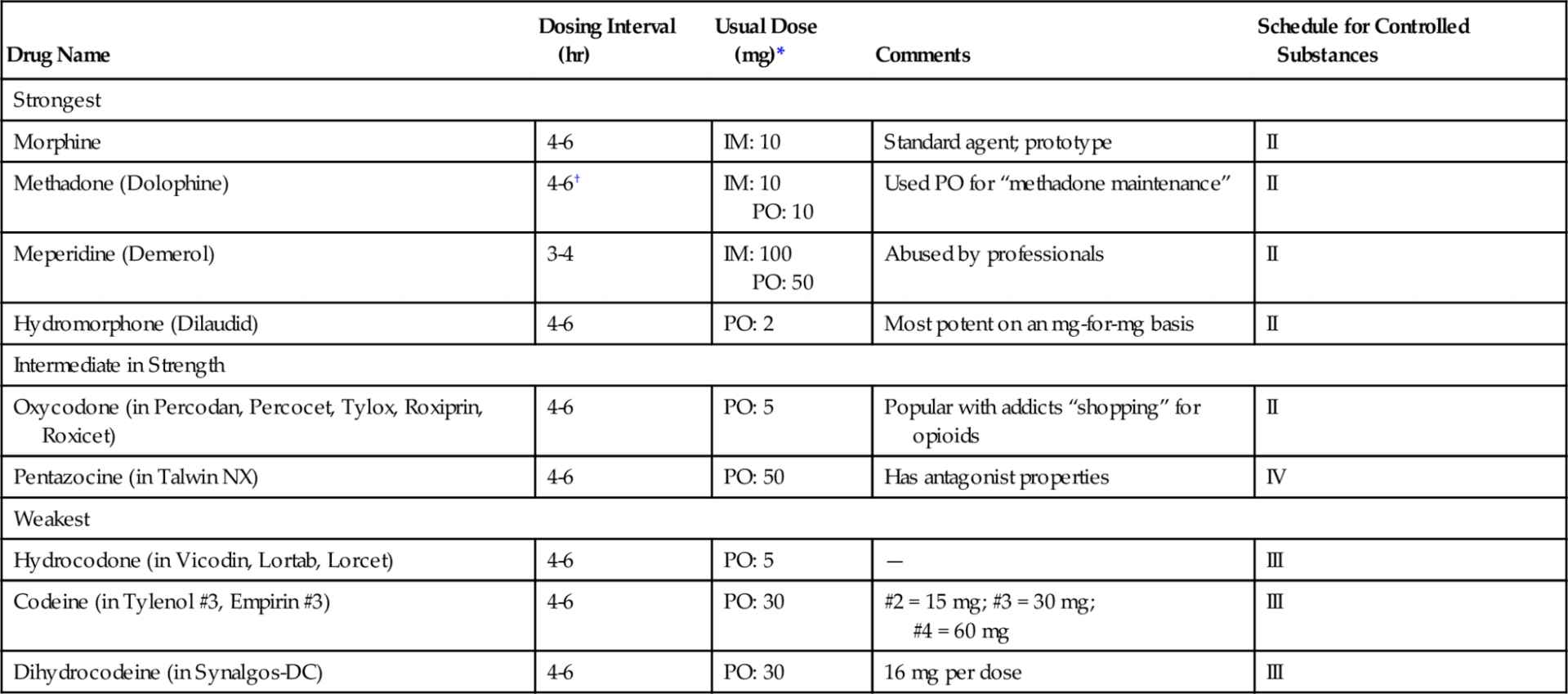
The dosing intervals and usual doses of some opioids are listed in Table 6-3. In general, onset occurs within 1 hour and duration necessitates dosing every 4-6 hours.
Pharmacologic effects
Although the pharmacologic effects and adverse reactions of the opioids are closely related, they are discussed separately. A pharmacologic effect may also be an adverse reaction, depending on the clinical use of the agent. In general, the severity of the side effects is proportional to the agent’s efficacy (strength).
Analgesia
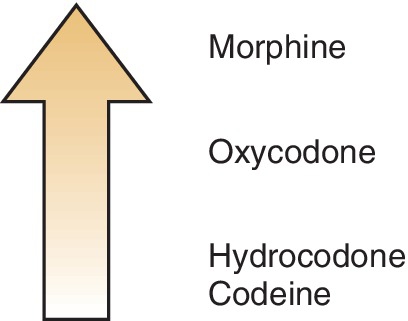
The opioid analgesics provide varying degrees of analgesia, depending on the strength of the agent. Figure 6-2 shows the relative analgesic efficacies of selected opioids. Morphine is the opioid agonist by which other opioids are measured. The strongest opioids can reduce even the most severe pain; the weaker agents mixed with nonopioids are equivalent to the NSAIDs in their ability to relieve pain; and the analgesic potency of the weakest agent (codeine) is low (see Table 6-3).
Codeine raises the pain threshold and affects the cerebral cortex to depress the reaction to pain. Both μ-receptors and κ-receptors are involved in producing analgesia. The opioids alter the patient’s reaction to painful stimuli, possibly by altering the release of certain central neurotransmitters.
Sedation and Euphoria
In the usual therapeutic doses, the opioid analgesics generally produce sedation by κ-receptor stimulation, which may potentiate their analgesic effect and relieve anxiety. This effect is additive with other CNS depressants such as alcohol. With larger doses, or if the pain is suddenly removed, euphoria can result. CNS excitation rarely occurs.
Cough Suppression
The opioids exert their antitussive action by depressing the cough center, located in the medulla. The dose that produces the antitussive effect is much lower than that required for analgesia, so the least potent agents are effective (e.g., codeine). Related compounds, such as dextromethorphan, are often used as antitussives.
Gastrointestinal Effects
The opioids increase the smooth muscle tone of the intestinal tract and markedly decrease its propulsive contractions and motility. This effect has made opioids useful in the symptomatic treatment of diarrhea. Opioid-like agents without analgesic properties, such as diphenoxylate (in Lomotil), are used to treat diarrhea.
Adverse reactions
Unlike the adverse reactions to many other drugs, the reactions to the opioids are not related to a direct damaging effect on hepatic, renal, or hematologic tissues but instead are an extension of their pharmacologic effects. Like the pharmacologic effects, the adverse reactions to the opioid analgesics are proportional to their analgesic strength. Table 6-4 lists contraindications to and cautions for the use of opioids.
Table 6-4
Contraindications to and Cautions for the Use of Opioids
| Condition | Comment |
| Alcoholism or addiction | Greater potential for abuse |
| Head injury | Can increase intracranial pressure |
| Chronic pain | Addiction potential limits (e.g., temporomandibular disease) duration |
| Respiratory disease | Respiratory depression can occur |
| Pregnancy | Respiratory depression near term (fetus) |
| Nursing | No problem: watch infant |
| Nausea | Additive nausea |
| Constipation | Exacerbates or produces constipation |
Respiratory Depression
The opioid analgesic agonists depress the respiratory center in a dose-related manner. This depression is usually the cause of death with an overdose. The depression is related to a decrease in the sensitivity of the brainstem to carbon dioxide. Both the rate and depth of breathing are reduced. In elderly or debilitated patients, the usual therapeutic dose of morphine can significantly decrease pulmonary ventilation. Reduced ventilation produces vasodilation, which results in an increase in intracranial pressure. Opioids should not be used in patients with head injuries. Opioids may also mask CNS diagnostic symptoms. Patients with hyperthyroidism are more tolerant of the depression, whereas patients with hypothyroidism are more sensitive to it.
Nausea and Emesis
Analgesic doses of opioid analgesics often cause nausea and vomiting. This is the result of their direct stimulation of the chemoreceptor trigger zone (CTZ), located in the medulla. This side effect is reduced if the patient does not ambulate. Administration of repeated, regular doses of an opioid can prevent vomiting by depressing the vomiting center (VC), another area in the CNS distinct from the CTZ.
Constipation
The opioids produce constipation by causing a tonic contraction of the gastrointestinal tract. Small doses of even weak opioids often have this effect, and their duration outlasts their analgesic effect. Even with continued administration, tolerance does not develop to this effect.
Miosis
The opioid analgesics cause miosis, an important sign (pinpoint pupils) in diagnosing an opioid overdose or identifying an addict. Tolerance does not develop to this effect.
Urinary Retention
The opioids increase the smooth muscle tone in the urinary tract, thereby causing urinary retention. They also have an antidiuretic effect through stimulation of the release of antidiuretic hormone (ADH) from the pituitary gland. This reaction may pose a problem in patients with prostatic hypertrophy.
Central Nervous System Effects
Occasionally, opioids may produce CNS stimulation, exhibited as anxiety, restlessness, or nervousness. Dysphoria can also occur from the opioids.
Cardiovascular Effects
The opioids may depress the vasomotor center and stimulate the vagus nerve. With high doses, postural hypotension, bradycardia, and even syncope may result.
Biliary Tract Constriction
In high doses, the opioids may constrict the biliary duct, resulting in biliary colic (pain associated with gallstones). This effect is important in patients passing gallstones who are being treated with opioids.
Histamine Release
Because the opioids can stimulate the release of histamine, itching and urticaria can result from their administration. This effect can occur at the site of intramuscular injection or at remote sites (e.g., itchy nose).
Pregnancy and Nursing Considerations
Opioids have not been shown to be teratogenic, although they may prolong labor or depress fetal respiration if given near term. The infant born to a mother using high-dose opioids, such as an addict, can have marked depressed respiration and experience withdrawal symptoms. The amount of opioid excreted in the mother’s milk when therapeutic doses are given to the mother would pose no problem to the normal infant. Morphine and codeine are classified as U.S. Food and Drug Administration (FDA) pregnancy category C. Acetaminophen is a pregnancy category B drug. Caution is urged because acetaminophen is often combined with opioid analgesics.
Addiction
Opioid addiction is a disease of the brain that involves both a physical dependence and a psychologic dependence on the drug. The two major signs of opioid addiction are cravings—an intense and overwhelming desire for the drug—and a loss of control of the ability to stop using the drug or to control the amount, which leads to a total loss of control. Both signs adversely affect the person’s life. Long-term administration of opioid analgesics can lead to tolerance, habituation, and dependence. Tolerance is normally not a problem for those who take opioid analgesics for no more than 1-3 days, as is the case in managing dental-related pain. Because the duration of use in dentistry is usually short, addiction does not often pose a problem for the dentist. NSAIDs should be used to control dental pain in the addict.
The degree of addiction
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses