Fig. 3.1
Example of MIH with molar as well as incisor opacities. Notice the white demarcated opacity 11, yellow demarcated opacity 21. Yellow brown demarcated opacities erupting 46, brown demarcated opacities with occlusal buccal posteruptive enamel loss 36, as well as demarcated yellow brown opacities in erupting 16 and 26 (Courtesy of Dr. H. Pohlen, Alsdorf, Germany)
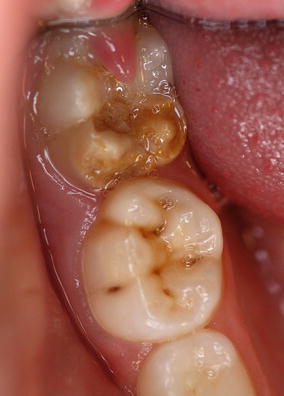
Fig. 3.2
Mild HSPM in the 85 while the erupting 46 shows already severe MIH (posteruptive enamel loss on both mesial cusps)
Diagnostic Criteria
In 2003, following a consensus meeting of the European Academy of Pediatric Dentistry (EAPD), criteria for epidemiological studies of MIH were developed including recognition of the potential for MIH-like hypomineralization defects to occur in second primary molars [4]. In 2009 in Helsinki, the EAPD updated these criteria [6] such that currently the criteria for MIH include demarcated opacities, posteruptive breakdown (or PEB) and both atypical restorations and extractions of the permanent molars and/or incisors. HSPM is defined similarly as hypomineralization of one to four second primary molars [7, 8]. To diagnose HSPM, the same criteria are used as for MIH with “atypical caries” being included in addition to “atypical restorations.” Especially in the primary dentition, cavities may not be restored in certain populations.
To arrive at a diagnosis of MIH, it is important to identify white, yellow or brown demarcated opacities on at least one first permanent molar. The greater the number of affected molars in an individual patient the greater the risk that the incisors will also be affected [2, 9]. The presence of opacities on the permanent incisors is not mandatory for the diagnosis of MIH. However, opacities that only occur on permanent incisors do not lead to a diagnosis of MIH. This is because the etiology of opacities confined to permanent incisors is likely to be different to that of the more widespread MIH condition. Traumatic injuries (particularly intrusive ones) to primary incisors impact on the developing tooth germs and commonly lead to localized anomalies of the permanent successors [2, 4, 10].
Clinical Features
MIH is a hypomineralization defect affecting the quality (as opposed to the quantity) of the enamel. It is identified visually as an alteration in translucency of the enamel, with a sharp demarcation between the affected and sound enamel, known as a demarcated opacity. The color of the hypomineralized area is white, yellow or brown and the area has a dull (porous) or shiny surface appearance (Fig. 3.3). MIH is not only variable in expression between patients but also within an individual patient. The number of affected first permanent molars per child can vary from one to four and the expression of the defects may vary from molar to molar. Within one patient, intact demarcated opacities can be found in one molar, while in another molar in the same patient, the porous enamel is already broken down (asymmetrical appearance). The porous brittle enamel can easily chip off under the masticatory forces very soon after eruption. This chipping off has been described in the literature as posteruptive enamel loss or PEB [4]. Sometimes the loss of enamel in PEB can occur so rapidly after eruption that it seems as if it was not formed initially (Fig. 3.4). However, it is important that the clinician distinguishes between PEB and hypoplasia. Hypoplasia is a primary quantitative defect of the enamel that occurs during tooth development. The resulting enamel may be thin or actually missing and may be characterized as pits or grooves across the tooth surface. The color and location of the demarcated opacities in MIH-affected molars are indicative of the relative risk for PEB; brown, dull, and porous opacities on cuspal tips are thought to be weaker than white and shiny ones on smooth surfaces and therefore more vulnerable to chipping [9].
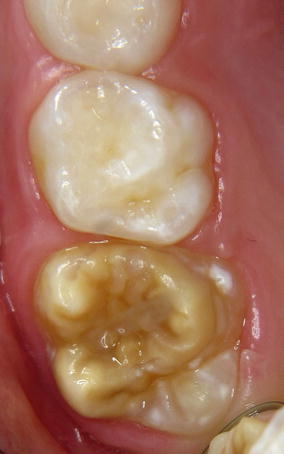
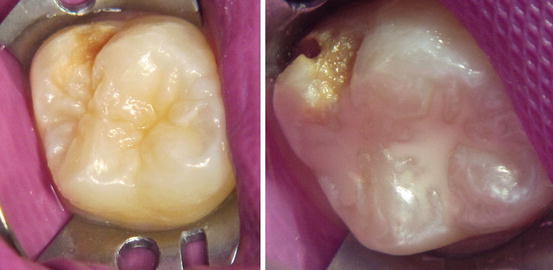

Fig. 3.3
Yellow to brown demarcated opacity covering almost the entire occlusal surface of the recently erupted 16 (Courtesy of Dr. M. van Ulsen, Amsterdam, the Netherlands)

Fig. 3.4
Tooth 16 with posteruptive enamel loss and opacity on the palatal surface. The same tooth after 1 year posteruptive enamel loss with cavity formation
The clinical appearance of the location, size, and form of the HSPM- and MIH-related carious lesions and restorations often do not fit with normal caries distribution patterns. These lesions and restorations have been reported as atypical caries or atypical restorations in some epidemiological studies [4]. When the incisors also show opacities most commonly these remain intact because the chewing forces have less impact on incisors compared with molars (Fig. 3.5).
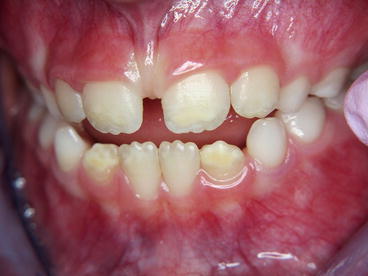

Fig. 3.5
Incisors with demarcated opacities in a child with severe MIH. Notice the white opacities in teeth 31 and 41, the light yellow/white opacities in teeth 11 and 21, and the dark yellow opacities in the 32 and 42
Both MIH and HSPM are very inconvenient for the child. The child may experience pain and sensitivity from the affected teeth particularly when exposed to hot and cold foods and drinks. Even if the (porous) hypomineralized enamel is intact, toothbrushing can cause sensitivity [2, 3]. In the event of PEB caries can progress rapidly increasing the risk of toothache. Pain-free treatment, which can be challenging because the teeth are sensitive, is important to avoid behavioral management problems or the development of dental anxiety in the future [11].
Etiology of MIH
Tooth development is under strict genetic control; however it is also very sensitive to environmental changes. Ameloblasts, which are highly specialized calcium-transporting cells, play an integral role in all three of the stages of enamel formation: matrix formation, initial calcification and final maturation [12]. If ameloblast function is disrupted during the secretory phase, there will be irreversible damage to the cells and the teeth are characterized by a deficiency of tooth substance that ranges from minor pits and groves to total absence of enamel; this is termed enamel hypoplasia and is a quantitative defect. If, however, disruption of ameloblasts occurs later during either the calcification or maturation phase, then the teeth will manifest in a qualitative defect termed enamel hypomineralization and can present as an enamel opacity [13].
As discussed in Chaps. 1 and 2, ameloblasts are highly susceptible to environmental disruptions leading to developmental defects in the enamel (DDE). Depending on the timing of the disruption, which can be from the time the first primary tooth starts to form (15–19 weeks in utero) to the completion of the maturation of the third permanent molar (around 12 years of age), the defects manifest differently across either dentition. However MIH is a very distinct form of DDE in which the enamel of the first permanent molars (+/− the incisors) is specifically hypomineralized. First permanent molars begin to develop in the fourth month in utero, but calcification does not start until around the time of birth. For an environmental disruption to contribute to MIH specifically, it would therefore likely occur from sometime around the time of birth to 3–5 years of age by which time maturation of the coronal enamel on the first permanent molars (and incisors) is complete. Recent in vitro studies have suggested an even narrower window of susceptibility around 6–8 months of age based upon the histological, mechanical, and chemical properties of affected first molar teeth [14, 15]. In general, the putative causes of MIH are similar to those identified in Chaps. 1 and 2 in association with more generalized DDE in both the primary and permanent dentitions. Table 3.1 is a summary of the possible etiological factors for MIH. The factors are divided into pre-, peri- and postnatal factors with medical problems being commonly mentioned. Fluoride does not appear to influence the occurrence of MIH [25].
Table 3.1
Factors putatively associated with MIH
|
Factor
|
Reference
|
||
|---|---|---|---|
|
Prenatal
|
|||
|
Lifestyle
|
Health
|
Maternal illness or infection
|
|
|
Maternal hypocalcemia
|
|||
|
Nutrition
|
|||
|
Perinatal
|
|||
|
Health
|
Infant hypoxia
|
||
|
Very low birth weight
|
|||
|
Premature birth
|
|||
|
Calcium shortage
|
|||
|
Misc medical problems
|
[18]
|
||
|
Postnatal
|
|||
|
Lifestyle
|
Breastfeeding
|
||
|
Nutrition
|
|||
|
Calcium shortage
|
|||
|
Environment
|
Dioxins and polychlorinated bisphenols
|
||
|
Environmental pollution
|
|||
|
Health
|
Childhood illnesses (in general)
|
||
|
Chicken pox and other viral infections
|
[17]
|
||
|
Otitis media
|
|||
|
Asthma, lung problems, allergy
|
|||
|
Fevers (irrespective of cause)
|
|||
|
Medications (in general)
|
|||
|
Antibiotics
|
|||
|
Antiasthmatic medication
|
[42]
|
||
The strength of the evidence surrounding the etiology of MIH specifically remains weak [18, 25]. There are a number of reasons for this including:
(i)
The historical lack of consensus surrounding both the definition of MIH and how to record it, leading to the use of a wide variety of often unvalidated indices.
(ii)
Reliance on retrospective data either using parental recall of the perinatal period or, slightly more reliable but nevertheless generally incomplete, retrospective chart reviews.
(iii)
Under-recording of the problem. First permanent molars affected by MIH are susceptible to both PEB and rapid carious attack shortly after emerging into the oral cavity. Many studies exclude restored or carious teeth from the assessment which is likely to lead to an under-reporting of MIH, while others may incorrectly classify PEB as hypoplasia. Similarly, severely affected molars often require early extraction. Many studies include population samples of children over 10 years old and few make any attempt to discover why missing teeth have been extracted – this is also likely to lead to under-reporting of MIH.
A further confounding factor when attempting to unravel the etiology of MIH is how to account for the typically asymmetric distribution of the hypomineralized lesions in MIH. This makes it difficult to attribute definitive causality between an environmental disruption and the presence of MIH. This may be explained in part by variations in the timing of mineralization of homologous pairs of teeth i.e. lower second molars [43]. It is also possible that there are genetic factors [44, 45] and local environmental factors within developing tooth germs themselves that may predispose to MIH. As understanding of the structure and composition of MIH-affected enamel evolves the etiology of this condition will become clearer but is almost certainly multifactorial and complex.
Etiology of HSPM
As with MIH, the etiology of HSPM appears to be multifactorial. Three studies have been published to date with the pre- and perinatal factors being reportedly more important in HSPM than in MIH [46–48]. Ninety-four percent of children with HSPM have been reported to have at least one medical problem: 24.5 % occurring prenatally, 45.3 % perinatally, and 9.4 % postnatally [46] with the greater the number of medical problems the greater the risk of HSPM. Furthermore, the ethnicity of the child, maternal alcohol consumption during pregnancy, low birth weight and fever episodes in the child’s first year of life have been identified as risk factors for HSPM [47]. Conversely, use of medications during pregnancy (specifically antibiotics and antiasthmatic and anti-allergic medications) does not seem to influence the occurrence of HSPM [47, 48].
Prevalence of MIH
The reported prevalence of MIH varies between 2.8 and 44 %. However despite the publication of recommended diagnostic criteria for [4, 6], the lack of consensus surrounding examination protocols, choice of index and population characteristics means that it is still hard to make valid comparisons between the various epidemiological studies [14, 25]. Table 3.2 summarizes the reported prevalence of MIH worldwide including the indices used in each study. In addition to differences in reported prevalence of MIH between countries, differences are also noted across birth year. For example, in the study of Koch et al. [1], the prevalence of MIH varied between 4.4 and 15.4 % across the different birth years with a peak in prevalence for those children born in 1970. These data were reproduced later by Jalevik et al. in Swedish children born in 1990 [26]. Most studies show an equal distribution of MIH between sexes [26, 55, 71] and, although Leppäniemi et al. [55] found a predisposition for MIH in the upper jaw, most studies fail to show any quadrant predilection for MIH defects [9, 26, 68, 71].
Table 3.2
Overview of the prevalence studies on MIH
|
Country
|
Prevalence (%)
|
Score criteria
|
Reference
|
|---|---|---|---|
|
Australia
|
22
|
mDDE
|
Arrow [49]
|
|
Australia
|
44
|
mDDE
|
Balmer et al. [50]
|
|
Bosnia and Herzegovina
|
12.3
|
EAPD 2003
|
Muratbegovic et al. [51]
|
|
Brazil
|
40.2
|
EAPD 2003
|
Soviero et al. [52]
|
|
Brazil
|
19.8
|
EAPD 2003
|
da Costa-Silva et al. [53]
|
|
China/Hong Kong
|
2.8
|
EAPD 2003
|
Cho et al. [54]
|
|
Denmark
|
37.3
|
EAPD 2003
|
Wogelius et al. [42]
|
|
Finland
|
17
|
Alaluusua 1996
|
Alaluusua et al. [30]
|
|
Finland
|
19.3
|
Alaluusua 1996
|
Leppaniemi et al. [55]
|
|
Germany
|
9.9
|
EAPD 2003
|
Petrou et al. [56]
|
|
Greece
|
10.2
|
EAPD 2003
|
Lygidakis et al. [16]
|
|
India
|
9.2
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


