Fig. 9.1
Surface of a clinically intact MIH lesion viewed under a scanning electron microscope showing characteristic surface irregularities (×1000 mag)
For qualitative defects, improving physical and chemical properties to optimize preservation and improvement of hypomineralized enamel tissue is the primary goal. Most strategies utilized clinically for hypomineralized enamel defects are based on the standard caries disease model, such as minimization of caries risk. However, some of these cannot always be applied directly to developmentally defective tissues because of the defective structure [2, 9]. Most particularly, in severe hypomineralization cases, intervention must be instituted as early as possible since significant damage can occur even before the crown is fully erupted (see Fig. 9.2). Anterior teeth are less affected by PEB and are less likely to become carious after enamel loss. However, molar teeth with PEB are at risk and should be closely monitored [10].
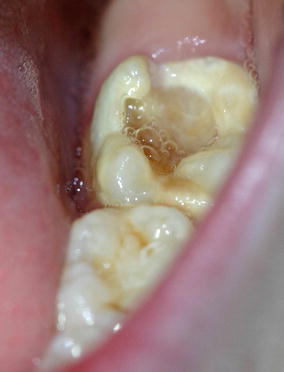

Fig. 9.2
MIH-affected tooth with severe loss of structure and caries before complete eruption
Prevention of Dental Caries and Erosion
To achieve success with any preventive strategy, early and accurate diagnosis is critical (see Chaps. 1, 2, 3, and 5). Once a diagnosis has been made, it is essential to emphasize the importance of general preventive principles for these teeth and to recognize that otherwise acceptable levels of care and compliance may be insufficient for caries prevention in these more vulnerable teeth. Where clinicians note that primary second molars or permanent incisors have DDE, this indicates the first permanent molars may also be affected and consideration should be given to altering recall timing and/or frequency to facilitate earliest possible intervention [11, 12]. If the case appears to be severe or complex, especially for genetic conditions such as AI or where sedation or general anesthesia may be required, early specialist referral to plan longer-term interventions may be the most appropriate management a general practitioner can provide (Chaps. 10 and 11).
Oral hygiene instruction should be undertaken including advice to ensure partially erupted teeth are cleaned appropriately – often using a buccolingual stroke with a soft brush for partially erupted molars to improve efficacy of plaque removal during the long eruption phase [13]. Given defects often manifest at an early age, before independent oral hygiene is recommended, the use of disclosing agents can assist both children and parents to better visualize deposits on the teeth. For sensitive teeth the simple suggestion to use warm water with the toothpaste when brushing may be helpful. Defects affecting the primary dentition may encourage increased cariogenic bacteria colonization with consequent higher caries risk. Although this has not been a consistent finding, it may still be prudent to include advice to reduce caries risk even when there has been little past evidence of caries [14, 15].
Dietary counseling, ideally in the form of a motivational interview where by providing information in a collaborative manner, encouraging willingness, ability, and readiness the clinician facilitates rather than directs change [16], is recommended [17]. This may include advice on caries prevention as well as on managing hypersensitivity, which is often as simple as avoiding or decreasing causative factors – i.e., cold drinks or food. As with standard dietary counseling, the role of fermentable carbohydrates (especially sucrose), particularly in terms of frequency and duration, should be discussed along with strategies to minimize exposures and ideas for healthier alternatives [18]. Intrinsic and/or extrinsic acids may also warrant some discussion not only in terms of exacerbating sensitivity but also, particularly in generalized conditions such as AI, because they may accelerate enamel loss in the form of erosive tooth wear. The influence of salivary characteristics should also be considered and potentially integrated into dietary counseling. As there is some evidence to suggest that salivary protection is less favorable in children affected by MIH, testing of salivary characteristics such as flow and consistency may be of benefit [14].
Preventive Agents
Another core component of caries prevention and desensitization is the use of fluoridated agents and calcium-based remineralizing agents. Ideally, for qualitative defects, the aim is not merely to remineralize any posteruptive demineralization but to address the primary mineral deficiency [19]. Studies of MIH, both in vivo and in vitro, suggest that casein phosphopeptide-amorphous calcium phosphate with and without fluoride (CPP-ACFP/ACP) application can improve enamel mineral content and reduce porosity both on exposed surfaces and deeper within the enamel tissue (see Fig. 9.3) [19, 20]. It is believed that a smoother surface, denser structure, and additional mineral content, especially if it is in the more chemically favorable forms of fluorapatite or fluoridated hydroxyapatite, should putatively reduce caries and breakdown risk [19].
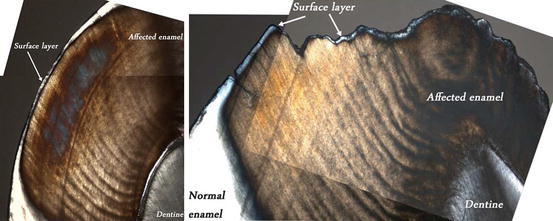

Fig. 9.3
Polarized light images of surface layer observed on both intact and PEB surfaces of MIH-affected teeth (in water, ×5 mag.). White/gray indicates reduced porosity (≤5 %), brown/orange indicates increased porosity (>5 %)
The action of CPP-based products relates to the ability of this milk-based protein to stabilize calcium and phosphate in high concentration and provide supersaturated ionic calcium and phosphate at and below the tooth surface. This then allows the diffusion of calcium and phosphate ions down a concentration gradient into the lesion at much higher concentrations than otherwise would be possible driving remineralization [21]. Reducing porosity is also likely to translate into a reduction in clinical sensitivity. Most reports of improvement in hypersensitivity are anecdotal; however, fluoride and remineralization treatments are consistently recommended in the literature, although a strong evidence base is lacking. There may be some additional benefit gained by prior deproteinization treatment; a similar finding has been reported for fluorosed enamel [19, 22]. In both of these cases, this was achieved by irrigation with a sodium hypochlorite solution (NaOCl); however, the clinical applicability/practicalities need to be considered given the likely difficulties in achieving adequate isolation and comfort. When NaOCl is used, the tooth needs to be completely isolated by sealed rubber dam, and as these teeth are often partially erupted, this may be difficult to achieve as may be adequate local analgesia. The leakage of the NaOCl into the oral cavity would have significant consequences.
Children with significant hypersensitivity or deemed at higher caries risk should be prescribed an optimal-strength fluoride toothpaste under appropriate professional and parental supervision. Anecdotally, frequent use of stannous fluoride has appeared to improve sensitivity in hypomineralized teeth; however, evidence is lacking. For very young children where controlling fluoride ingestion may be difficult, a calcium phosphate-based product would be recommended initially in conjunction with professional application of a fluoride varnish or a stannous fluoride gel. It seems likely that the greatest gains of continuing mineralization will be made as the teeth emerge. Like demineralization lesions, hypomineralized enamel defects have been shown to develop a thin but relatively well-mineralized surface layer which helps prevent further loss of minerals but can inhibit subsequent, deeper tissue improvement in mineral content (see Fig. 9.4) [6].
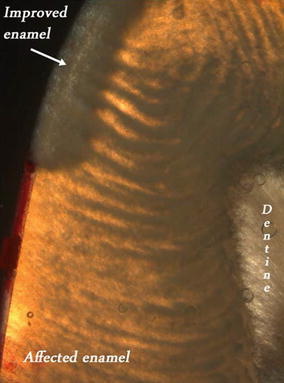

Fig. 9.4
Polarized light image of an MIH lesion following exposure to a CPP-ACFP solution in vitro (in water, ×5 mag.). Brown/orange area is the unchanged lesion (>5 % porosity), gray area (5 % porosity) shows the part of the lesion exposed to the solution, while the red surface coating protects the unexposed (control) part of the lesion
Surface Sealing
Sealants
In more severe hypomineralization cases, preventive measures alone are often insufficient and some form of surface protection is indicated [23]. At its simplest this will be standard sealing and this is recommended prior to full emergence of the tooth [24]. A glass ionomer cement (GIC) material is therefore the best initial choice as it can tolerate the presence of some moisture and can provide a source of ions which appear to be advantageous to the underlying tooth in several ways. An incidental finding of an MIH characterization study suggested that deeper mineralization improvements occur under a GIC sealant compared with enamel surfaces exposed to the oral environment [25]. It has been reported that GIC materials also provide a “halo protection” effect, whereby healthy enamel immediately adjacent to a GIC material is less susceptible to demineralization, which may be further enhanced by the addition of CPP-ACP to the GIC [26]. Where PEB has already occurred, protective sealing may be extended over all the affected areas to decrease caries risk until such time that more definitive treatment can be undertaken. Where sealing cannot be achieved, full coverage options should be considered (Chaps. 10 and 11). Once isolation can be established, there are more clinical options available (Chap. 11). However, resin-based sealants are not always recommended because of the difficulties in bonding to enamel that is hypomineralized and has a higher protein content (see Chap. 6). It is widely reported that enamel defects display atypical and often poor etch patterns (Chap. 6) with implications for bonding of resin materials including resin sealants [27–30]. Extended etching times for severely fluorotic enamel are recommended because of the increased resistance of fluorapatite to acidic dissolution [27]. There is some evidence to suggest that the higher organic content of defective enamel limits remineralization and reduces bond strengths. Techniques such as irrigation with NaOCl can remove protein and improve bonding and penetration of resin materials in MIH and AI [29, 31–33]. It appears important that to increase bond strengths, the NaOCl should be used after etching, not before [29, 34, 35].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


