Fig. 7.1
The effect of rubber dam on lightness can be observed in these three photographs. (a) Patient’s smile before rubber dam isolation. (b) The rubber dam was placed in the upper dental arch and left undisturbed for 10 min. (c) The effect of dehydration is observed immediately after rubber dam removal (Images provided by Camilo Andrés Pulido Mora, DDS, MS)
Apart from dehydration, enamel demineralization results from the low pH of most bleaching products currently available. Most in-office bleaching gels are delivered in low pH because they are more stable in acid solutions than in basic solutions. When HP is to be stored, a weak acid is usually added to the solution to prevent it from decomposing (Chen et al. 1993), which makes the bleaching product acidic enough to produce enamel demineralization. The pH of in-office bleaching gels may vary from 2.0 to 9.0 (Price et al. 2000; Freire et al. 2009; Majeed et al. 2011).
Therefore, taking into consideration that transient dental dehydration and demineralization occurs concomitantly to the permanent effect of dental bleaching during in-office bleaching, the result of in-office bleaching cannot be assessed immediately after the in-office bleaching session. The reliability of color measurements is questionable if carried out immediately after treatment, leading to the conclusion that in-office whitening is as efficient as at-home bleaching.
The effect of color change when evaluated before complete dental rehydration occurred in the study of Matis et al. (2007). The graphic below shows the changes in L* (Fig. 7.2) and b* (Fig. 7.3) parameters after a single in-office bleaching session. In the “x” axis, time 0 means the color taken immediately after in-office bleaching and the other times represent weekly measures up to 6 weeks. In general, color change (L* and b* parameters) seems much more pronounced when they were measured immediately after the procedure (time 0), with significant reductions of L* and increases of b* after 1–2 weeks due to the dental rehydration and remineralization. Therefore, the “real” bleaching effect (produced by oxidization) can only be measured 1–2 weeks after the end of the in-office bleaching.
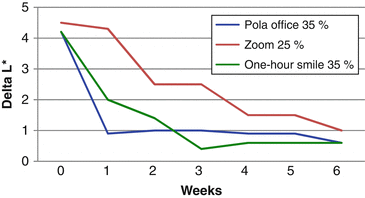
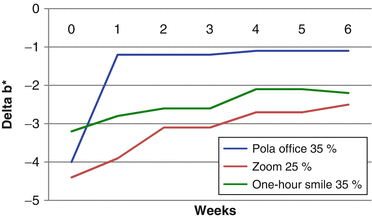

Fig. 7.2
Variation in the ΔL* in a 6-week period after a single in-office bleaching with different products (Adapted from Matis et al. 2007)

Fig. 7.3
Variation in the Δb* in a 6-week period after a single in-office bleaching with different products (Adapted from Matis et al. 2007)
The difference between the “whitening outcome” observed immediately after bleaching and that measured 1 week later has been erroneously interpreted as color rebound, with some researchers concluding that in-office bleaching is not as efficient as at-home bleaching (Matis et al. 2007). Several studies have demonstrated that 1 week of at-home bleaching with 10 or 16 % carbamide peroxide gel usually results in a change of two to four shade guide units in the value-oriented Vita Classical A1-D4™ shade guide (VITA Zahnfabrik H. Rauter GmbH & Co.KG, Bad Säckingen, Germany) (Zekonis et al. 2003; Bernardon et al. 2010; da Costa et al. 2010; Rezende et al. 2013). This is approximately equivalent to the change reported after a single in-office bleaching session with 35 % HP gel when used for 45–60 min (Zekonis et al. 2003; Bernardon et al. 2010; Kossatz et al. 2011; Reis et al. 2011b).
There are also other factors that may explain the general belief that in-office bleaching is not effective. It is known that the whitening effect is related to the concentration, application time, and the number of changes of the in-office bleaching gel (Dietschi et al. 2006; Joiner 2006; Matis et al. 2007). In an ongoing systematic review of the literature (Luque-Martinez et al. 2016) we observed a high heterogeneity among studies in many issues, such as type of materials, concentration of the products, and significant variations in in-office bleaching protocols.
Inefficient bleaching protocols will not lead to a satisfactory bleaching outcome. For instance, some studies performed only a single in-office bleaching session (da Costa et al. 2010; Giachetti et al. 2010; Moghadam et al. 2013; Pintado-Palomino et al. 2015), which is not enough to reach patient’s satisfaction (de Silva Gottardi et al. 2006; Salem and Osman 2011). At least two or three bleaching sessions may need to be performed to obtain a similar whitening degree of a 2 or 3-week at-home bleaching (Marson et al. 2008b; Tay et al. 2009; Bernardon et al. 2010; Basting et al. 2012; Reis et al. 2011b, 2013).
Similar variation occurs in regard to product application time. While a 40–50-min application is related with a significant whitening outcome, there are reports of shorter application times, such as 10–20 min (Auschill et al. 2005; Giachetti et al. 2010; Mehta et al. 2013). A very recent clinical study reported that a single 15-min application of the 35 % HP does not achieve the same degree of whitening produced by two and three 15-min applications of the same product (Kose et al. 2015).
Some manufacturers advocate the application of their products with light activation (quartz–tungsten halogen light curing units, LEDs, and lasers) to optimize the bleaching outcome (Ziemba et al. 2005; Kishi et al. 2011; Bortolatto et al. 2014). The benefits of this association are rather controversial (Buchalla and Attin 2007; He et al. 2012), but it seems to be useless for high-concentrated HP gels (Marson et al. 2008b; Alomari and El Daraa 2010; Kossatz et al. 2011; He et al. 2012). For low-concentrated HP gels this light association may have some benefits, but this still requires further evaluations (Ziemba et al. 2005; Ontiveros and Paravina 2009; Bortolatto et al. 2014). This will be discussed in more detail in the section of frequently asked questions in this chapter.
This variation makes the comparison of the in-office bleaching protocols very difficult. However, efficient whitening has been observed in studies that employed 35 % HP, with reports of overall color change of five to eight shade guide units after two in-office bleaching sessions (Marson et al. 2008b; Tay et al. 2009; Bernardon et al. 2010; Strobl et al. 2010; Reis et al. 2011a). This wide range of color change probably is the result of the small variations in the HP concentration, number of bleaching sessions, and baseline color of the participants in the clinical trials (Rezende et al. 2015b).
7.3 Adverse Effects
As in-office bleaching is used with higher HP concentrations, there are more concerns about adverse effects in comparison with at-home bleaching. The two most frequent adverse effect of in-office bleaching is bleaching-induced tooth sensitivity (TS) and gingival tissue burning.
7.3.1 Bleaching-Induced Tooth Sensitivity (TS)
While effective bleaching is reported to occur with in-office bleaching, several publications have reported that patients undergoing bleaching procedures frequently complain of painful and uncomfortable sensations arising in the treated teeth. Although pain in bleached teeth can be evoked by cold or other stimuli, most patients complain of tingling or shooting pain (zingers) of very short duration but variable frequency (Haywood 2005) without provoking stimuli (Markowitz 2010).
Unfortunately, this side effect is very frequent. The reported risk of bleaching-induced TS in clinical trials of dental bleaching is quite variable but easily exceeds 50 %. A recent study that evaluated the individual patient data of 11 clinical trials regarding bleaching produced a more accurate estimate of these risks. For in-office bleaching in higher concentration (35 %), the risk of TS was reported to be 62.9 % (95 % CI 56.9–67.3), which was not too different from that reported for 10–16 % carbamide peroxide for at-home bleaching (51 % with a 95 % CI 41.4–60.6) (Rezende et al. 2015b). Although the risk of TS was reported to be similar, the intensity of TS was very different between bleaching protocols. In a 0–4 pain scale, the overall mean intensity of bleaching-induced TS for in-office bleaching was 2.8 ± 2.9, while for at-home bleaching was 0.5 ± 0.9 (Rezende et al. 2015b).
The etiology of bleaching-induced TS is not fully understood. Since the hydrodynamic theory of dentin sensitivity has been widely accepted as the explanation of dentinal sensation, some authors have used this theory to explain bleaching-induced TS (Swift 2005). However, pain during and following bleaching treatment can affect intact teeth lacking dentin exposure and this is in sharp contrast with the hydrodynamic theory (Markowitz 2010).
In face of that, other investigators have hypothesized that bleaching-induced TS may result from some degree of pulpal inflammation due to the higher amount of HP that reaches the pulp. It is widely known that HP can pass easily through the enamel and dentin to the pulp (Cooper et al. 1992) and can cause damage to the pulp cells as seen in Chap. 5 (Costa et al. 2010). Further proof of this passage of HP is the fact that color changes in dentin next to the pulp occur as fast they do at the dentin–enamel junction (McCaslin et al. 1999; Haywood 2005). Pulp tissue damage is likely to lead to the release of cell-derived factors, such as adenosine triphosphate (Cook and McCleskey 2002) and prostaglandins, which excite or sensitize pulpal nociceptors (Huynh and Yagiela 2003) causing the bleaching-induced TS (please refer to Chap. 5).
Several factors may affect the ability of HP to permeate the dental structures and consequently the damage produced by the bleaching gels. For instance, the amount of HP that permeates dental pulps is higher in teeth with restorations (Gokay et al. 2000; Patri et al. 2013; Parreiras et al. 2014). In restored teeth, the depth and size of the restorations (Parreiras et al. 2014), as well as the type of adhesive and restorative material (Gokay et al. 2000), may also play a significant role on the amount of HP penetration.
The tooth type is another important factor. Literature findings report that for the upper dental arch (Bonafe et al. 2013), the tooth that was reported to give most complaints of bleaching-induced TS was the upper lateral incisor. The thinner enamel and dentin layers of incisors compared to other teeth may allow the fast passage of HP to the pulp, allowing less time for the production and release of protective enzymes against damage by HP. This was also in agreement with recent histological studies of human pulps after in-office bleaching (Costa et al. 2010; Roderjan et al. 2015). In one study (Costa et al. 2010), the authors observed notable damage to the pulp tissue of lower incisors but not to premolars (Chap. 5).
Baseline color was strongly associated with TS in a recent study that pooled the data from 11 studies from the same research group (Rezende et al. 2015b). In other words, the darker the teeth, the lower the intensity and risk of TS. Darker teeth probably have higher organic content to retain the HP in the enamel and dentin substrates, allowing less surplus HP to travel to the pulp tissue. Under these circumstances, it is possible that less HP comes in contact with the pulp tissue, which generates lower TS. This, however, is a hypothesis yet not supported by basic research.
Several approaches have been tested to minimize the adverse side effect of TS. The administration of some drugs perioperatively during in-office bleaching, such as selective anti-inflammatory drugs (etoricoxibe) (de Paula et al. 2013), nonsteroid anti-inflammatory drugs (ibuprofen) (Charakorn et al. 2009; Paula et al. 2013), antioxidants (ascorbic acid) (de Paula et al. 2014), and corticoids (dexamethasone) (Rezende et al. 2015a), was not effective to prevent the risk as well as the intensity of TS as confirmed by a recent systematic review of the literature (Faria et al. 2015). Under per-oral administration, several factors such as the immune system, lymphatic drainage, urinary excretion, and morphological characteristics of the dentin substrate may modulate the amount of the medicine that reaches the plasma and extracellular fluid around pulp cells, making these approaches not effective.
The most effective measures to minimize this side effect were through the application of topical desensitizers (Wang et al. 2015). The preoperative application of a gel composed of 5 % potassium nitrate and 2 % sodium fluoride for 10 min was capable to reduce the risk of TS by half, as well as the intensity of TS (Tay et al. 2009). The effect of fluoride in this process is not clear and the desensitizing effect of the association of sodium fluoride and potassium nitrate seems to be more related to the presence of potassium nitrate. This substance penetrates the enamel and dentin to travel to the pulp where it creates a calming effect on the nerve by affecting the transmission of nerve impulses (Ajcharanukul et al. 2007). After the nerve depolarizes in the pain stimulus response, it cannot repolarize, so the excitability of the nerve is reduced. Potassium nitrate has almost an anesthetic effect on the nerve (Haywood 2005).
In regard to the action of fluorides, it is hypothesized that the precipitation of calcium fluoride crystals in dentin can reduce the functional radius of the dentinal tubules and also the permeability of this tissue to the hydrogen peroxide. By doing so, less hydrogen peroxide reaches the pulp chamber, reducing the tooth sensitivity. This, however, is yet to be confirmed, as this process seems to occur only when there are exposed dentin surfaces.
Another study showed that previous desensitization with Gluma desensitizer (Heraeus Kulzer), composed of 5 wt% glutaraldehyde and 35 % weight% HEMA (Baba et al. 2002; Qin et al. 2006), for 1 min significantly reduced sensitivity of the anterior teeth during and after whitening compared with a placebo pretreatment (Mehta et al. 2013). The authors of this study hypothesized that glutaraldehyde (molar mass 100 g/moL) and HEMA (molar mass 130 g/moL) might penetrate through enamel and dentin along the same pathway as the peroxide radicals. On the way to the pulp, glutaraldehyde might react by cross-linking with enamel matrix proteins and with proteins in the dentin tubular liquid, thus reducing easy passage of the HP radicals to the pulp.
Although some opinion leaders claim that application of desensitizer gels prior to in-office bleaching affects the bleaching efficacy, this was not confirmed by recent meta-analyses of the literature (Wang et al. 2015), probably because the desensitizer gels used are transparent.
7.3.2 Gingival Tissue Irritation
As long as adequate protection of the gingival tissues is performed with a light-cured gingival barrier or rubber dam isolation, gingival burning (Fig. 7.4) is not expected to occur. This is usually not reported in clinical trials of in-office bleaching studies and reflects the clinical experience of the study authors. As seen in Chap. 4, in case this occurs the dentist should apply a drop of catalase and/or sodium bicarbonate (usually provided by the manufacturer) on the ulcerated lesion to arrest the burning effect. No other measure is usually required; but in case the patient feels any discomfort, a corticoid ointment may be prescribed to relieve pain.
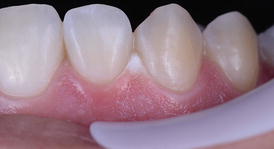

Fig. 7.4
Chemical burning of the cervical gingiva of several teeth after an in-office bleaching application with a high-concentration hydrogen peroxide
7.4 Treatment Regimen with Step-By-Step Procedures
As mentioned earlier, in-office bleaching protocols vary significantly in the clinical reports. The application time of the bleaching gel, the number of bleaching sessions, whether or not the protocol is associated with light and the number of product refreshment on the dental surface are some examples.
In this chapter, we will describe all steps involved in an effective bleaching protocol and also report some of the variations of each step as long as they can still result in an effective whitening outcome. For didactic reasons, this section will be described in steps.
7.4.1 Making a Decision About the In-Office Bleaching Gel
There are many in-office bleaching products in the dental market, which makes their choice quite difficult. They vary in the active concentration of HP, which ranges usually from 15 to 40 % and in terms of pH (Freire et al. 2009; Price et al. 2000; Majeed et al. 2011). There are some products that contain other additives such as calcium gluconate and calcium phosphates and desensitizing agents (sodium fluoride, potassium nitrate). These systems also vary in their mode of application: most of them require product refreshment during a single in-office session, while for some products; a single 40–50-min application is required.
The literature is scarce regarding the comparison of these systems both in terms of effectiveness and side effects, and, therefore, the choice of these products is usually based on empirical evidence. Comparison of in-office bleaching gels with different HP concentrations is also scarce. A single study that compared the color change and bleaching-induced TS of 20 % versus 35 % HP with 2 % calcium gluconate reported no significant difference in the risk of TS and a significant lower degree of whitening for the 20 % HP gel (Reis et al. 2013).
As previously mentioned, whitening products should have a relatively alkaline pH to minimize potential damage, but there is a wide pH variation among in-office bleaching gels (Price et al. 2000; Freire et al. 2009; Majeed et al. 2011). This variation could be the result of the different formulations used by each manufacturer, because bleaching agents contain stabilizers and other inorganic components that allow them to be stored for prolonged periods. In-office bleaching gels are delivered in low pH because they are more stable in acidic solutions than in basic solutions. When the HP is manufactured, a weak acid is usually added to the solution to prevent it from decomposing (Chen et al. 1993).
Some investigators have reported that the HP delivered in an alkaline medium increases the effectiveness of bleaching in the wool industry. This effectiveness is explained by the fact that the dissociation constant of the HP is about 11.5. In fact, the findings of one study showed that in a pH = 9.0, the dissociation rate of the HP was 2.7 times higher than that in an acidic solution (pH = 4.4) (Frysh et al. 1995) and this was recently confirmed by Torres et al. (Torres et al. 2014). They observed in vitro that the efficacy of hydrogen peroxide bleaching is directly proportional to the increase of the pH of the bleaching gel. These variations, however, did not seem to produce differences in tooth-bleaching effectiveness when products with acidic and alkaline pH were compared, although a significant decrease of tooth sensitivity has been shown for alkaline gels (Kossatz et al. 2012).
Additionally, it is worth mentioning that alkaline gels usually show more stable pH during application than acidic gels (Marson et al. 2008a), which allows them to be applied in a single application without the need of several product replenishments (Reis et al. 2011a, b; Kossatz et al. 2012).
Although there is biological plausibility to choose bleaching products containing desensitizing agents such as potassium nitrate, to the best of the authors’ knowledge no randomized clinical trials have compared the TS levels produced by in-office gels with and without desensitizer agents. Only at-home clinical studies evaluated this hypothesis (Navarra et al. 2014; Gallo et al. 2009) as previously mentioned in the Chap. 6.
In summary, we recommend the use of 35 % alkaline gels, containing desensitizing agents. As mentioned in the section on frequently asked questions, reduced HP concentration can be used in the combined or jumped-started technique. In regard to the presence of desensitizing agents, we still recommend products containing it. The absence of evidence that desensitizing containing gels can reduce TS cannot be interpreted as evidence of absence of an effect. These studies are usually low powered and we cannot rule out the fact that desensitizing-containing gels can provide some beneficial effect. Until high-powered studies are published, we should work in the conservative way and use such type of products, as they do not have any known detrimental effects. Finally, products should be applied according to the respective manufacturer’s instructions.
7.4.2 Determination of the Baseline Tooth Color
This procedure allows dentist and also the patient to monitor color change during the bleaching protocol (Fig. 7.5). Patients usually very quickly get used to the new tooth color and may not remember what color their teeth were before protocol. This is even more important when both dental arches are bleached simultaneously. Shade recording can be a procedure with a value-oriented or bleach shade guide (Fig. 7.5), spectrophotometer, or by means of dental photographs.
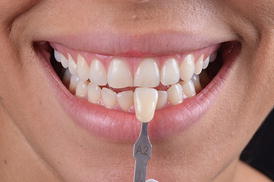

Fig. 7.5
The baseline tooth color being recorded with a value-oriented shade guide after performing a dental prophylaxis
Some authors encourage whitening one dental arch at a time (Haywood 2005), because it minimizes TS, allows the patient to monitor the opposing arch to compare progress, and it also encourages compliance. However, this procedure increases significantly the cost of the bleaching protocol, as it requires more dental visits.
Another advantage of color recording is that baseline dental color can predict the whitening degree obtained after dental bleaching. A recent multivariable regression analysis (Rezende et al. 2015b) identified a significant relationship between baseline color and age in relation to color change estimates. After adjustment for the other variables, every increase of one shade guide unit (in the value-oriented Vita Classical A1-D4™ shade guide) in the baseline color resulted in an increase of approximate 0.66 in the final color change in ΔSGU and 2.48 for the ΔE, meaning that the darker the baseline tooth color, the higher the degree of whitening. In an opposite trend, the degree of whitening is negatively affected by the participant’s age (Rezende et al. 2015b).
This allows for the dentist to manage the patient’s expectations in regard to the bleaching outcomes. Older patients with lighter baseline color may request more than the two bleaching sessions to achieve the same whitening degree than younger patients with darker baseline dental color.
It is important to perform a dental prophylaxis recording the baseline tooth color. A recent published paper showed a significant difference (average of two ΔE units of change) on tooth color when measured before and after dental prophylaxis. This may reach the threshold for clinical detection (ΔE = 3.0) for some patients (de Geus et al. 2015).
7.4.3 Application of a Desensitizing Agent
As reported earlier, one of the main side effects of in-office dental bleaching is TS. Although this side effect cannot be completely eliminated, the number of patients that experience TS and the intensity of TS can be reduced by previous application of a desensitizing gel composed of 5 % potassium nitrate (Tay et al. 2009; Wang et al. 2015). Desensitizers composed of glutaraldehyde and HEMA was also reported to be effective to reduce the bleaching-induced TS, and can be an alternative to the potassium nitrate gel (Mehta et al. 2013).
This procedure can be performed before or after isolation of the dental arch, as the material is not aggressive to the gingival tissue. However, as the gel is usually agitated with the aid of a rotating brush it is recommended to apply the desensitizer before the protection of the soft tissues. The buccal surface of all teeth to be bleached should be covered with a 1-mm thick layer of the desensitizer and left in place for at least 10 min (Fig. 7.6). At the end of this period, the product should be agitated in each dental surface for 20 s with a rotating brush before removal. The inclusion of this step into the in-office bleaching protocol does not jeopardize the whitening efficacy of the hydrogen peroxide (Tay et al. 2009). After this period, the product should be removed with gauze (Fig. 7.7) or with a saliva ejector before application of the in-office bleaching gel. Rinsing can be performed as a final step for complete removal of the product.
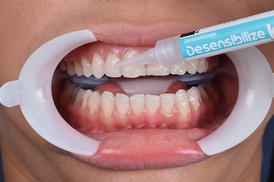
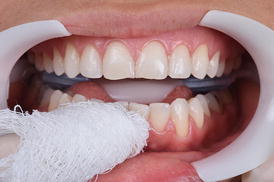

Fig. 7.6
Application of a desensitizing gel composed of 5 % potassium nitrate for 10 min (Dessensibilize KF 2 %, FGM, Joinville, SC, Brazil). After this period, the product should be agitated in each dental surface for 20 s with a rotating brush before removal

Fig. 7.7
Removal of the desensitizing gel with dental gauze or high-speed suction. After removal of the excesses, water rinsing was performed
7.4.4 Protection of the Soft Tissues
Hydrogen peroxide in high concentrations, such as those used for in-office bleaching, may cause burning of the dental tissues (Fig. 7.4). Several attempts should be made to avoid contact with the soft tissues.
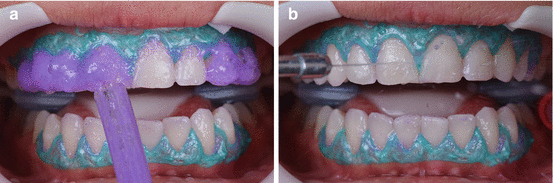
The use of lip and cheek retractors associated with a light-cured gingival barrier (Fig. 7.8) is quite common. The former can maintain lips, cheeks, and even tongue away from the bleaching gel while the latter prevents the contact of the bleaching gel with the gingival tissue. An increased frequency of micronuclei of cells from the gingival tissue (which is an evidence of genotoxicity) was observed in patients submitted to in-office bleaching (Klaric et al. 2013), which may be the result of soft burning or even the contact with the gingival barrier. To avoid this, the light-curing gingival barrier should be adequately light cured (Fig. 7.8), according to the respective manufacturer’s recommendations, and clinicians should look at the teeth from an incisal aspect to detect any sealing failure of the gingival tissue.
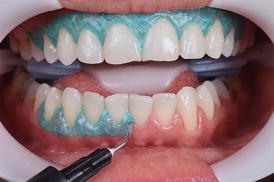

Fig. 7.8
A lip and cheek retractor (ArcFlex, FGM, Joinville, SC, Brazil) is applied, followed by the application of a light-cured gingival barrier to protect the marginal gingival tissue
Rubber dam isolation can also be used for protection of the soft tissues. However, before rubber dam installation, a thick layer of petroleum jelly should be applied on the gingival tissue of the teeth to be bleached. Due to the hydrophobic nature of the petroleum jelly, the bleaching gel will be prevented from contacting the gingival tissue even if eventual isolation failure occurs.
7.4.5 Application of the In-Office Bleaching Gel
After choosing the in-office bleaching product, the manufacturer’s instructions should be followed (Fig. 7.9 and 7.10). Variations to what is advocated by manufacturers may lead to either whitening at reduced speed or increased TS rates (Reis et al. 2011b; Kose et al. 2015). By increasing the number and/or time of application, one may increase the degree of whitening obtained but at the time the risk of TS is also increased. In an opposite trend, reducing the number and/or time of application reduces the probability of TS but also limits the degree of whitening.
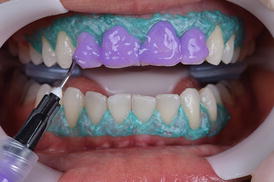
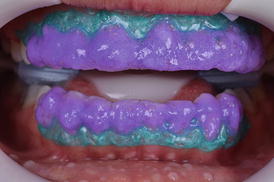

Fig. 7.9
The 35 % hydrogen peroxide in-office bleaching gel (Whiteness HP Blue 35 %, FGM, Joinville, SC, Brazil) is mixed and applied in all teeth to be bleached

Fig. 7.10
After some time in place, bubbles are visible in the gel, which result from the decomposition of the hydrogen peroxide
Most in-office bleaching gels require replenishing the product during a period that varies from 40 to 50 min. Some products require two, three, or four product replenishments in each clinical session. There are some products, however, that are indicated for a single 40–50-min application without replenishment. These products usually possess a basic pH that allows them to be used for longer application times without increasing the risk of TS (Kossatz et al. 2012; Reis et al. 2013). The product should be firstly removed with a cotton pellet, gauze, or high-speed suction (Fig. 7.11) before rinsing the dental surfaces with water. This procedure prevents any kind of soft tissue burning.