Fig.1
(a) developing tooth in a bud stage, (b) developing tooth in a cap stage, (c) developing tooth in a bell stage
During the cap stage (Fig. 1b) (at about 9th–10th week), 3 basic structures develop, i.e. the enamel organ, dental papilla and dental follicle.
The bell stage (Fig. 1c) (approx. 11th–12th week) is primarily characterized by the activation of the enamel organ and the formation of the dentino-enamel junction. The enamel organ is composed of 4 epithelial layers—the outer enamel epithelium (OEE), reticular epithelium, intermediate stratum and inner enamel epithelium (IEE). The IEE starts to differentiate into preameloblasts which induce the proliferation of ectomesenchymal cells in the outer layer of dental papilla and their differentiation to (pre)odontoblasts. Odontoblasts start to secrete predentin which induces the process of preameloblasts maturation into ameloblasts, and so the secretion of the enamel commences. When the amelogenesis (i.e. the production and full mineralization of the enamel) is finished, the ameloblasts degenerate and disappear.
Later in the bell stage, the cervical loop (an area of the enamel organ located on the future crown-root border, composed of the IEE and the OEE) elongates apically through proliferation towards the epithelial diaphragm and eventually becomes Hertwig’s epithelial root sheath (HERS). ) The HERS then initiates the tooth root formation and activates the production of root dentin (in the same manner as in the crown region). After the activation of the local odontoblasts the root sheath disintegrates, this way the newly generated dentin becomes exposed to the ectomesenchymal cells in the inner layer of dental follicle, which eventually induces the differentiation of these follicular cells into cementoblasts and so the production of precementum. The exposed dentin further induces the differentiation of fibroblasts from the middle layer of dental follicle and so eventually the creation of periodontal ligament, and the differentiation of osteoblasts of alveolar socket leading to the creation of the outer layer of the dental follicle.
Eventually, the mature soft dental-related tissues are dental pulp, periodontal ligament, gingiva and apical papilla, and the mature hard dental-related tissues are enamel (oral epithelium), dentine and cementum (neural-crest-derived ectomesenchyme).
Dental-Related Tissues’ Stem. Cell Lineages Differentiation Potential and Anatomic Location
Tissues’ stem cell populations quality and quantity vary with their origin and stage of maturation. At maturation, the hard dental tissues posses no stem cell populations, while the soft dental-related tissues do and facilitate both self-regeneration as well as regeneration of them adjoined hard tissues [13, 14]. The only hard dental tissue which lacks the possibility to repair is enamel for the absence of communication with any soft tissue.
For the summary of up-to-date differentiation potential of dental-related stem cell lineages see Table 1.
Table 1
Differentiation potential of dental-related tissues stem cells lineages
|
Differentiation capacity
|
DPSC
|
iRPSC (SCAP)
|
iTDPSC(germ)
|
NDP-SCs
|
SHED
|
DFSCs
|
PDLSCs
|
GMSCs
|
|---|---|---|---|---|---|---|---|---|
|
Adipogenic
|
Struys [15]
|
Sonoyama [16]
|
Yalvac [17]
|
Akpinar [18]
|
Miura et al. [6]
|
Kémoun [19]
|
Xu [20]
|
Gao et al. [21]
|
|
Chondrogenic
|
Koyama [22]
|
Wang [23]
|
Demerci [24]
|
Akpinar [18]
|
Kerkis [5]
|
Kémoun [19]
|
Xu [20]
|
El-Sayed [25]
|
|
Osteogenic
|
Mori [26]
|
Bakopoulou et al. [27]
|
Yalvac [17]
|
Akpinar [18]
|
Miura et al. [6]
|
Morsczeck [11]
|
Xu [20]
|
Gao et al. [21]
|
|
Neurogenic
|
Osathanon [28]
|
Sonoyama [29]
|
Yalvac [17]
|
Karaöz [30]
|
Miura et al. [6]
|
Morsczeck [11]
|
Shi [31]
|
Zhang et al. [32]
|
|
Myogenic
|
Zhang [33]
|
Abe et al. [34]
|
Tasli [35]
|
Karaöz [30]
|
Kerkis [5]
|
d’Aquino [36]
|
Shi [31]
|
Ansari [37]
|
|
Endothelial cells
|
Hilkens [38]
|
Bakopoulou [39]
|
Yalvac [17]
|
Cordeiro [40]
|
Dögan [41]
|
Zhang et al. [32]
|
||
|
Odontogenic
|
Huang [42]
|
Sonoyama [29]
|
Demerci [24]
|
Miura et al. [6]
|
Chen [43]
|
Shi [31]
|
Gao et al. [21]
|
|
|
Hepatogenic
|
Ishkitiev [44]
|
Patil [45]
|
Ikeda [46]
|
Ishkitiev [47]
|
Patil [45]
|
Kawanabe [48]
|
||
|
Cementogenic
|
Kim [49]
|
Kim [49]
|
Kémoun [19]
|
Seo [9]
|
||||
|
Melanocytes
|
Paino [50]
|
|||||||
|
Cardiomyocytes
|
Armiňán [51]
|
|||||||
|
Epithelial cells
|
Dögan [41]
|
|||||||
|
Endodermal cells
|
Zhang et al. [10]
|
|||||||
|
Periodontal ligaments
|
Morsczeck [11]
|
|||||||
|
Pancreatic islets
|
Ishkitiev [52]
|
|||||||
|
Cornea cells
|
Gomes [53]
|
Tooth Organ-Stem Cell Lineages
Immature Tooth Organ-Stem Cell Lineages
Dental Papilla Stem Cells: iTDPSC
Immature Tooth Dental Papilla is an engineering tissue of mesenchymal origin enclosed by the enamel organ on the top and by the dental follicle on the bottom, which eventually converts to pulp tissue. It plays central role in epithelial-mesenchymal interactions responsible for tooth morphogenesis. It is firstly responsible for the crown morphogenesis and later, when it becomes apical to dental pulp (a converted top part of dental papilla, after the onset of dentinogenesis/bell stage) it becomes secondly also responsible for the morphogenesis of the tooth roots (in the form of Apical Papilla) [54–56]. The most suitable teeth for obtaining dental papilla are third molars where the roots are not developed at all, this condition is fulfilled before the 14 years of age (Figs. 2 and 3a).
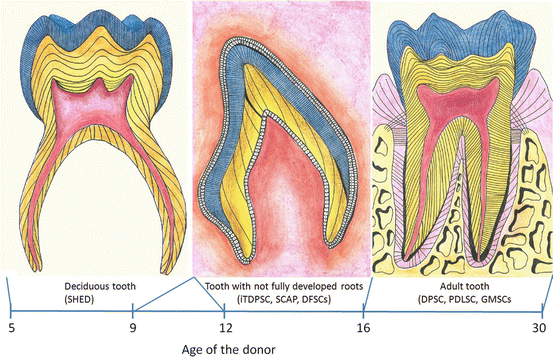
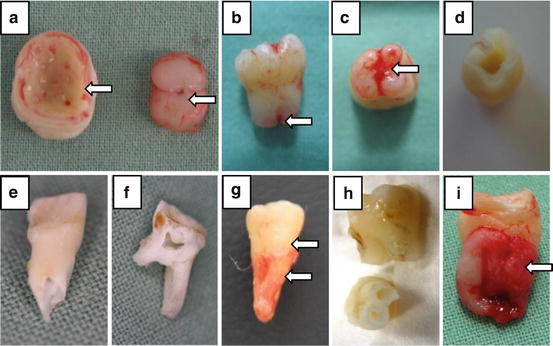

Fig. 2
Dental related stem cells lineages and the most common age of the donors of the different stem cells populations

Fig. 3
(a) Extracted 3rd lower molar in a bell stage (germectomy) on the left side and its pulp on the right side. (b) Extracted 3rd lower molar with not fully developed roots. (c) Extracted 3rd lower molar with not fully developed roots with the apical papilla covering the root orificium. (d) Extracted 3rd lower molar with not fully developed roots from the (c) after extraction of the pulp tissue. (e) Extracted upper deciduous first incisor where the root apex is reabsorbed and the root canal widely opened. (f) Extracted second lower deciduous molar after splitting the crown using the bur to open the pulp chamber. (g) Extracted upper 3rd molar, the lower arrow indicates the area which is covered by the periodontal ligaments and presents the suitable source of the periodontal ligament stem cells, the upper arrow indicates the cement-enamel junction. (h) Two extracted upper first premolars after the isolation of the dental pulp. (i) Extracted lower 3rd molar with dental follicle tissue marked with the arrow
The up-to-date knowledge of the differentiation potential of the Dental Papilla Stem Cells is minimal and calls for further research. Most recently, in 2014, Vishwakarma et al. have stated that the dental papilla stem cells “can induce both enamel-dentine and dentine-cementum production”, but only under certain specific conditions and combined with the appropriate cell populations.
Apical Papilla Stem Cells/Immature Root Papilla Stem Cells: SCAP/iRPSCs
The root formation of developing tooth starts when the cervical loop-epithelial cells proliferate apically and induce the differentiation of dental follicle mesenchymal cells into odontoblasts and cementoblasts [57] This apically extending epithelial wall forms Hertwig’s epithelial root sheath (HERS) which guides the shape of the tooth roots. It is at this time dental papilla becomes apical to dental pulp tissue (hence apical papilla) and forms apical cell-rich zone as a divide between the two [55]. Overall, apical papilla is histologically different from dental pulp of immature tooth in multiple ways, according to Sonoyama et al., its stem cells proliferate 2 to 3 times quicker than those of the adjoined immature dental pulp, it contains less cellular and vascular components and it further contains unique potent MSCs. As apical papilla is a tissue derived from the neural crest, its stem cells express numerous neurogenic markers [58].
Mature Tooth Organ-Stem Cell Lineages
Dental Pulp
Dental pulp originates in dental papilla and is made up of connective tissue and four distinct cell-zones. The dental pulp connective tissue consist of an extracellular matrix (ECM) composed of ground substance with relatively high content of glycosaminoglycans, proteoglycans and other adhesion molecules (fibronectin, laminin, etc.) and the sparsely distributed type III collagen fibres which form a rich network only around vessels and nerves. The outmost cell-zone of the dental pulp is the odontoblast layer-zone (gives rise to the primary “predentine”, secondary and tertiary “reparative” dentine), followed inwards by a cell-free-zone called the Weil basal layer (contains dense capillary and nerve network), then cell-rich-zone, and finally arriving at the pupal core. The last two zones share similar structure as they are composed of larger vessels, nerves and great amount of cells, specifically: fibroblasts, cells belonging to the defense system, and undifferentiated mesenchymal stem cells.
Dental pulp stem cells lineages’ quality and characteristics differ with dentition (predeciduous, primary, secondary) and level of their maturation.
Predeciduous Dentition Dental Pulp Stem Cells/Natal, Neo-Natal Dental Pulp Stem Cells NDP-SCs
The natal (erupted before birth) and neonatal (erupted within the days after birth) teeth are rarity found in some newborn babies within the first few days after birth. Natal teeth are more frequent than neonatal teeth, with the ratio being approximately 3:1 [59]. These teeth are usually indicated for extraction due to the danger connected to their inhalation, the risk that they could cause deformity and mutilation of tongue, dehydration, malnutrition, growth retardation of the following dentitions and due to the health risks they can cause to the mother during breastfeeding. Histologically, the natal teeth have only thin or completely absent layer of both enamel and cementum. The predentin layer is often of various thickness compiled of irregular dentinal tubules. Furthermore, unlike dental pulp of teeth of the primary and secondary dentitions, the natal dental pulp has extra inflammatory areas and does not have the Weil basal layer and cell-rich-zone. These teeth tend to have a wide pulp chamber and underdeveloped roots which makes isolation of their dental pulp easier.
Primary Dentition Dental Pulp Stem Cells: SHED
The amount of stem cells from human exfoliated deciduous teeth decreases with the receding dental pulp (which is gradually replaced by gum). This implies that the deciduous teeth that fall out spontaneously are likely to have little to no pulp, therefore little to no SHED population. To ensure a sufficient amount of stem cells will be isolated, the rule of thumb is to focus on teeth which have at least 1/3 of the original root length (after the onset of primary dentition root resorption), and for the multi-rooted teeth it is best to isolate teeth with the furcation area still present (Fig. 3e, f).
The ideal time to extract the deciduous teeth for their dental pulp stem cells is between the ages of 5 and 9. Beware; dental pulp of teeth affected by dental carries is not fitting for use in research.
Secondary Dentition Dental Pulp Stem Cells: DPSCs
The teeth most commonly used for isolation of the dental pulp stem cells are the first premolars and the third molars (Fig. 3g, h), as they frequently attract preliminary extraction at early age of the dental tissue. The first premolars are usually extracted shortly after eruption, due to the orthodontic reasons, around the age of 12. The “youth” of their soft dental-tissues, not fully developed root, and very low probability of carries makes these teeth the ideal stem cell source. The third molars are often extracted to prevent health complication such as the surrounding tissue inflammation, as well as due to the orthodontic reasons. While the extraction for orthodontic reasons comes around the age of 16, the extraction due to tissue inflammation occurs later, usually between the ages of 16–30, hence involves at least partially-erupted and often fully developed tooth.
Tooth-Supportive Tissues-Stem Cell Lineages
Immature Tooth-Supportive Tissues-Stem Cell Lineages
Dental Follicle Stem/Progenitor Cells: DFSCs
The dental follicle is a loose connective tissue that surrounds the developing tooth and is separated from dentin by an epithelial layer (Hertwing’s sheet). Once this epithelial layer disintegrates and dental follicle touches the dentin, it is prompt to differentiate into periodontium including the alveolar bone, cementum, and periodontal ligament [60]. According to Vishwakarma et al. [61], the tissue contains at least three distinct stem cell populations (hDF1, hDF2, hDF3) with distinct morphologies, gene expressions and differentiation potentials.
Most commonly, the unerupted third molars are used for isolation of these stem cells, usually before 14 years of age (Fig. 3i).
Mature Tooth-Supportive Tissues-Stem Cell Lineages
Periodontal Ligament Stem Cells: PDLSCs
The periodontal ligament connects alveolar bone and cementum to support teeth in situ and preserve the hard tissues homoeostasis [9]. Periodontal ligament stem cells are commonly isolated from the soft tissue adjoined to the root below its enamel-cementum border (Fig. 3g); they form adherent clonogenic population of fibroblastic-like cells, forming flat and loose aggregates [62]. In 2010, Kawanabe et al. [62] have shown that PDLSCs express embryonic stem cell-associated antigen SSEA-4, implying these stem cells are great source of building material for regenerative medicine, being capable of differentiating into cells of all three germ layers.
The periodontal ligament tissue is usually isolated from around the first premolars and third molars from donors who have no history of periodontal disease and healthy periodontium.
Gingiva-Derived Mesenchymal Stem Cells: GMSCs
The gingiva is a mucosal soft tissue which forms a shield-like physical protection of the adjoined dental-related tissues by sealing the gap between tooth’s exposed enamel and periodontal ligament protected cementum; it further exhibits both immunomodulatory and anti-inflammatory capacities, which allow it to help the adjoined tissues to regenerate [10].
The GMSCs are usually derived from the spinous layer of human gingiva [64]. This highly homogenous population of stem cells carries not only the usual mesenchymal stem cell markers but also a positive expression of extracellular matrix proteins [10]. GMSCs have been successfully differentiated into osteoblasts, chondrocytes, adipocytes.
Donor
Theoretically any vital tooth and its enveloping tissues provide possible source of dental-related stem cells. However, it is important to narrow the pool of possible donors to those who are most likely to donate viable and research feasible stem cell populations, will be the least impacted by the extraction procedure, populations, while undergo the least impactful extraction procedure. Eventually, electing the right research material is directly related to choosing a fitting donor.
The three basic recommendations in choosing the right donor:
- 1.
Dental-related tissues to be extracted
-
Different stem cells lineages can only be isolated from donors of certain age/development status
-
- 2.
Health status of the donor
-
General medical history—as certain illnesses and diseases (e.g. genetic predispositions) can cause researcher bias as they may influence the qualities of the research material
-
Possible impact of tissue extraction on the donor’s health
-
- 3.
Clinical status of the tissue
-
The general condition of the tissue and its immediate environment represent yet another research bias (e.g. caries lesions represent risk of bacterial transmission, inflammation of the soft tissue can adversely impact the research material, erupted tooth is contaminated by the oral micro microflora, fully developed roots complicate the process of the dental pulp isolation)
-
Election of Research Method
Bioethics, Legislation and Regulation of Human Stem Cell Research
The principal goal of human biomedical stem cell research is to relieve and avert suffering caused by both genetic and acquired impediments to health condition [65].
Due to the interdisciplinary character of the knowledge base and the worldwide network of research which aims to build upon each other, while oftentimes towards different goals, it is eminently important to unify the standards for information integrity, full disclosure, and respect for research participants—their health and freedoms [65]. While the research focused on the unipotent, multipotent and pluripotent stem cells avoids the major ethical and legal issues connected to the totipotent embryonic stem cells and oocytes, there are other concerns which remain standing.
The international consensus over the stem cell research guidelines is conveyed by the ISSCR—International Society for Stem Cell Research, established independent non-profit organisation founded in 2002, which releases yearly updated guidelines for stem cell research and clinical trials and provides templates of legal documents connected to biomaterial donation and transfer [65]. The ICSCN—International Consortium of Stem Cell Networks then provides the contacts and means to bridge varying national stem cell legislation and regulation to foster best practice international research collaborations. The consortium is managed by a secretariat comprising of nationally funded representatives from Australia, Canada, Germany and Scotland and meets annually in conjunction with ISSCR.
Beyond the international organisations, the research ethics and regulations are further narrowed by supra-national consortia such as the European EuroStemCell and the American USFDA—Food and Drug Administration of the United States of America ; national legislation and regulations issued by National Academies; and eventually by the institutional SCRO—Stem Cell Oversight Committee [66]. The SCRO must respectively consist of proxies of both scientific and ethical communities (also referred to as: IRE—Institutional Review Board/IEC—Independent Ethics Committee/ERB—Ethical Review Board or REB—Research Ethics Board), from which a part is non-affiliated. The ethical community further demands membership of representatives of ethical expertise and those representing the lay public [67]. Finally, the text summarising the institutional professional standards and self-regulations, issued by the institutional SCRO, deals with all the above issues in two separate parts: Part 1: Respect for research participants and Part 2: Integrity of the research enterprise [67]. The SCRO standards for non-totipotent stem cells research should, according to the ISSCR 2015 Draft Guidelines [65] and Hyun [68], address the following:
- 1.
Respect for Research ParticipantsThe respect for research participants remains accompanied with the issue of donor/patient consent to share genetic information and the issue of donor/patient mental and physical state now and in the future; which prohibits scientists to treat the biomaterial freely and subject it to downstream research which has not been approved by the SRCO and the donor in advance [68]. The above issues require written and legally valid informed consent to donate (if the donor is not sui juris, their legal representative has to subscribe the informed consent instead), which according to the ISSCR Draft 2015, should address both the donor and research organisations’ rights and obligations towards each other, specifically:
- (a)
The provision of accurate information on risks to donor health connected to tissue retrieval.
- (b)
The provision of accurate information on risks, limitations, possible benefits, and available alternatives to patient treatment.
- (c)
The donated tissue treatment and storage—genetic modification, recipient profile, discard protocol, ad cetera.
- (d)
The genetic and disease screening of the donated material.
- (e)
The full disclosure of the derived information.
- (f)
The donor/patient private information (identification, genomic information, ad cetera) treatment and discard protocol.
- (g)
The future accessibility of the donor/patient to obtain additional consent, additional material or information.
- (h)
The commercial potential of the donated material and intellectual property rights of the researchers/donor.
- (i)
The treatment of incidental findings.
- (j)
The treatment and legal liability of the researchers and medical staff in case of unforeseeable impacts on patient health [65].
- (a)
- 2.
Integrity of the Research EnterpriseThe treatment of the biological material founded upon valid, timely, reliable and reproducible protocol; executed following the GLP—Good Laboratory Practices; and reported in transparent, reliable manner and accessible format ensures integrity of research enterprise. All of the above is pertinent for independent peer review and downstream research.
The US FDA provides a step-by-step toolkit to institutional SCRO organisation, operation, administration and reporting which can be used as the building blocks of the institutional standards [65].
Dental-Related Tissue Extraction and Transport
Dental-Related Tissues Extraction
The extraction of tissues for stem cell research is performed under the same condition as an ordinary prescribed extraction, while we are interested in preserving the biological material as intact as possible, the health of the donor/patient comes first. Fundamentally, there are two types of extraction, the simple extraction and the surgical extraction; where both are performed under local anesthesia.
The simple extraction is adopted in the case of fully erupted tooth and is executed using various dental tools including elevator and forceps to break the tooth’ bonds to periodontal ligament and to widen its alveolar bone socket, and so allow for smooth removal of intact tooth.
The surgical extraction is adopted in case of an impacted or not yet fully erupted tooth (the most common source of dental-related stem cell material) (Fig. 4). This procedure involves rising of mucoperiosteal flap/tissue flap on the intra-oral side of gums covering the tooth (for esthetic reasons) and trimming the exposed bone present around the tooth roots to allow for smooth root removal (here the prime consideration is to limit the amount of bone being removed). At this point, depending on situation, the surgeon either retains the tooth as a whole, which is preferable, or sections it to further ease its extraction. Once sectioned, however; the dental-related tissues are compromised together with their value in research.
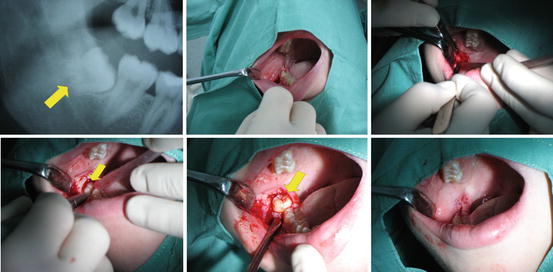

Fig. 4
The procedure of complicated extraction of lower 3rd molar. First picture represent the cut of the ortopantomogram. Second and third pictures show the moving of the mucoperiostal flap. On the fourth picture the bone covering the tooth is removed and on the fifth picture we can see the extraction using the levers
Immediately after the extraction it is recommended to disinfect the tooth using any disinfectant solution commonly used in the dental cavity, to decrease the possible amount of bacterial presence. Then, the tooth needs to be fully immersed in the Hanks balanced salt solution enhanced by antibiotics and antifungals (composed of 1 ml of Hanks balanced salt solution, 9 ml water for inj., 200 μl/10 ml gentamycin, 200 μl/10 ml streptomycin, 200 μl/10 ml and 200 μl/10 ml penicillin) to fully eliminate any remaining bacterial presence.
Dental-Related Tissues Transport
The temperature of the solution during transport is highly recommended to be kept at 4 °C. Even though stem cells are theoretically resistant to hypoxia and survive longer than somatic cells, it is absolutely essential they are isolated from the extracted tissues as soon as possible (no later than 24 h after extraction). For this reason it is necessary to invest in proper preliminary planning to avoid any wastage.
Isolation of the Dental-Related Tissues
Tooth-Organ Tissues Extraction
Immature Tooth-Organ Tissues Extraction
Dental Papilla and Apical Papilla Extraction
Dental papilla tissue is extracted from tooth without developed roots, hence sourced from very young donors/patients. Such extraction is called germectomy (Fig. 3a) (an identical procedure is used to obtain dental follicle tissue (Fig. 3i). The most common source of the dental papilla tissue for stem cell research are third molars of donors/patients aged 10–14 years, as those teeth are commonly indicated for extraction. After the onset of root dentine formation and root development, the dental papilla splits into the soft tissues of dental pulp and apical papilla.
Apical papilla is localized at the area of root growth, below dental pulp (Fig. 3b–d). The dental pulp and apical papilla are clearly separated by apical papilla adjoined apical cell-rich zone which however does not stick to dental pulp and so allows smooth separation of the two tissues.
Whilst dental and apical papilla tissues can be obtained from immature teeth of both dentitions, it is rare to find immature deciduous teeth indicated for extraction which have no caries lesion; therefore the SCAP and iTDPSC are widely considered as stem cell lineages of secondary dentition.
Mature Tooth-Organ Tissues Extraction
Dental Pulp Extraction
Dental pulp consists of connective tissue and odontoblasts locked together inside the dental pulp chamber. During the process of tooth maturation, dental pulp tissue becomes increasingly enclosed in hard dental-related tissue, until only a tiny opening at the roots’ apex (physiological apical foramen) is left (0.25–0.35 mm wide at maturation) to allow the nervous connections and blood vessels. To ensure the extracted dental pulp tissue is as intact as possible, we aim to use the least destructive method which varies with the tooth level of maturation (including all postnatal immature and mature dental pulps of all dentitions).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


