Fig. 5.1
Surface energy measured by the OWRK method of turned, micro-textured, and nano-textured Ti-6Al-4V substrates (Source: Paulo G. Coelho’s laboratory archives)
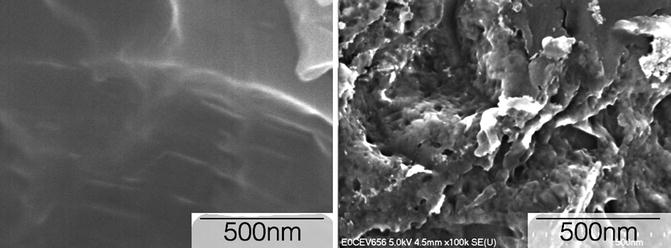
Fig. 5.2
High-resolution FE-SEM micrograph of a (left) micrometer scale textured surface and (right) a nanometer scale textured surface (Source: Paulo G. Coelho’s laboratory archives)
The positive effect of surfaces presenting nanometer scale features on the adhesion, spreading, motility, proliferation, adhesion selectivity, and differentiation of osteoblasts has been previously compiled [18–22]. Thus, there is unequivocal evidence that cells can be triggered through nanometer length scale modifications and that such modifications should be incorporated into dental implant designing to support the bone response.
From a hierarchical implant design perspective, the implant hardware and microgeometric/topographical role is to not only provide the device primary stability through mechanical engagement and an increase in friction between implant and bone, respectively, but also to allow adequate bone healing conditions where substantial interaction between blood clot and implant surface exists immediately after placement [23]. While implant hardware and micrometer scale modifications will dictate tissue-level interaction and healing pathway cascade around the implant [6, 8, 23, 24], nanometer scale features present potential in boosting osteoblastic behavior and thus supporting the bone response. However, while in vitro cell culture studies demonstrated positive effects of nanometer scale features on osteoblastic cells [25–27], direct translation of nanometer scale features to implants, without considering implant hardware features and micrometer scale design parameters, may not relate with laboratory in vivo findings. Such contradiction led to the rationale for the hierarchical placement of nanometer scale features within micrometer scale texturing when designing dental implant surfaces. Such strategy attempts to assure adequate intimate interaction between blood clot and the implant surface so osteogenic cells may travel through a seamless pathway towards the device surface to be further altered in phenotype by nanometer scale features. Under this design guideline, implant surfaces presenting nanometer scale texture and chemistry alteration have been manufactured.
Commercially Available Implant Surfaces Presenting Nanometer Scale Features
Reduced scale manufacturing techniques have been compiled in the engineering literature and are beyond the scope of this chapter. From a manufacturing perspective, many if not all of the available techniques may be utilized for patterning implant surfaces with nanometer scale features [14]. However, since high throughput is necessary for economically viable implant surface manufacturing, industrial methods for nanometer scale surface modification are restricted to a reduced number of additive and subtractive methods. To date, four representative implant surfaces presenting nanometer scale features have been made commercially available. Out of these four, two comprise surface modifications presenting calcium-and-phosphate bioactive components manufactured through initially subtractive methods followed by additive methods, and the other two are manufactured mainly through subtractive processes.
The nanometer scale surfaces presenting bioactive ceramic components were both named NanoTite™ by their respective manufacturers (Bicon LLC, Boston, USA; Biomet 3i, Palm Beach Gardens, USA). While their final physicochemical configuration is distinctively different, both surfaces are manufactured by an initial subtractive method prior to the additive methods that are distinct. For the Bicon surface, a 20–50 nm-thickness ion beam-assisted deposition (IBAD) of calcium phosphate results in the coating of a moderately rough micro-textured substrate obtained by alumina blasting/acid etching (AB/AE) [28]. For the Biomet 3i surface, a sol–gel process is performed for the deposition of calcium phosphate nanometer-sized (called discrete crystalline deposition, DCD) particles onto their textured acid-etched surface. Previous work has demonstrated that the particle component covered approximately 50 % of the surface [29]. While both surfaces presented bioactive components on their surfaces, the rationale for their presence and intended coating kinetics in vivo substantially differed.
The rationale for the IBAD of 20–50 nm mainly amorphous coating thickness onto the AB/AE micro-textured surface was to take advantage of the highly osteoconductive properties of the calcium phosphate coating properties while avoiding long-term issues presented by thick plasma-sprayed hydroxyapatite coatings in which long-term performance is highly dependent on implant hardware configuration [30]. In short, the basis was to provide the healing site with the known osteogenic properties of bioactive calcium phosphate elements, and due to the IBAD coating amorphous configuration, the coating would be entirely dissolved/resorbed from the surface and an intimate contact between bone and the AB/AE micro-textured surface would result. Different from the IBAD coating, the DCD method for nanometer scale feature incorporation intended to increase the substrate osteoconductivity due to the multiscale texture levels (micro- and nano-texturing) and chemical composition rendered by the DCD method.
The two other surfaces presenting nanometer scale features are both manufactured by subtractive methods [31]. The first surface, OsseoSpeed™ (Astra Tech, AB, Mölndal, Sweden), from here on referred by the OSP acronym, initially utilizes a titanium oxide blasting procedure that renders the surface with micrometer-level texture, followed by a hydrofluoric acid etching procedure that results in nanometer scale texturing within the micrometer scale texturing. The second one, OSSEAN™ (Intra-Lock International, Boca Raton, FL, USA), from hereon referred to by the OSS acronym, is fabricated by robotic microblasting of a resorbable blasting media powder that simultaneously results in nanometer scale topography within the larger-scale micro-topography. Regardless of fabrication method, no long-range ordering of the nanometer scale features is obtained [31, 32].
Despite the substantially different fabrication methods, from a topographical standpoint, all four surfaces present nano-texture within micro-textured surface features. From a surface chemistry standpoint, the IBAD fabricated surface presents primarily Ca, P, and O in its surface since it is a uniform coating at the nanometer length scale thickness, whereas the DCD surface presents elements from both bioactive ceramic and substrate components [29, 33]. Along with the substrate alloy components, fluoride presence at minute quantities has been detected on OSP [34], whereas bioactive ceramic along with substrate alloy elements is present on the OSS surface [35].
It must be stated here that some of the implant surfaces other than the abovementioned were discovered afterwards by scientific reports to possess nanoscale features. For example, TiUnite (Nobel Biocare, Zurich, Switzerland), SLActive (Institut Straumann, Basel, Switzerland), and OsseoSpeed (DENTSPLY IH, Mölndal, Sweden), all of them, which were originally not claiming of possessing nanoscale features, were discovered to have distinctive nano-patterns [2, 36, 37]. Whether intended or not intended, these features may be one of the reasons for the enhanced bone apposition observed when compared to their respective predecessors.
Biological Response to Nanotextured Implant Surfaces Made Commercially Available
Despite the recent introduction of the nanometer scale onto implant surfaces, a substantial body of work has been developed. The early studies investigating the biological response to nanometer scale surfaces were funded by manufacturers and often utilized their predecessor surfaces as control groups. A series of studies followed and at times different nanometer scale surfaces were compared within studies.
Cell Culture Studies
The IBAD surface was evaluated in three different cell culture studies, where the IBAD surface was compared to its AB/AE uncoated substrate and as-machined surfaces [38–40]. The first study, carried out with primary human osteoblasts, presented mixed results between the IBAD and AB/AE surfaces regarding events related to osteogenesis [38]. The second study aimed to evaluate the same three surfaces under human osteogenic cells, peripheral blood mononuclear cells (PBMC), and osteogenic cells cocultured with PBMC without exogenous stimuli. In general, relative differences in results were observed between surfaces for three different cultures (always favoring the IBAD and AB/AE surfaces relative to the as-machined control); however, the “multicell type” interactions played a more remarkable role than the surface texture or chemistry on the in vitro cellular events related to initial stages of bone formation [40]. Finally, the same three surfaces were evaluated in human polymorphonuclear neutrophil (PMN) culture. The results showed that the addition of a thin CaP coating to the AB/AE surface influenced the secretion profile of proinflammatory cytokines [39]. Altogether, cell culture studies including the IBAD surface presented mixed results that demonstrated substantial disagreement with the in vivo preclinical results between IBAD, AB/AE, and turned surfaces as subsequently discussed.
The DCD surface has been evaluated in primary mouse alveolar bone cells relative to Astra OsseoSpeed, Nobel Biocare TiUnite, and Straumann SLA surfaces [41]. The results following a 48-h culture, Astra and Straumann systems displayed the highest degrees of cell adhesion. Specific to the DCD surface, it presented significantly lower degrees of cell confluence relative to the other surfaces [41].
The OSP surface has been compared to its titanium oxide blasted predecessor in a mouse preosteoblast MC3T3-E1 cell culture model [42]. The study results showed no differences in cell viability and proliferation but more branched cell morphology was observed for OSP relative to the control at 48 h. At 14 days, increased degrees of IGF-I, BSP, and osterix gene expression were observed for the OSP surface, concluding that osteoblast differentiation and mineralization were affected by the nanometer scale surface [42]. A more comprehensive real-time PCR study considering bone-specific gene expression in the same two surfaces plus a turned control in a MC3T3-E1 cell culture model and in implant adherent cells from a rabbit tibia model depicted that OSP surface outperformed the control surface in osteogenic gene expression events [43]. Another study evaluating adherent mesenchymal stem cells on OSP and its predecessor presented favorable osteoinductivity and osteogenesis of these cells for the OSP surface [44]. As previously mentioned, when OSP was compared to the DCD surface in a primary mouse alveolar bone cell culture model, favorable results were observed for the OSP surface relative to the DCD surface [41]. Masaki et al. also demonstrated that OSP altered cell behavior relative to other surfaces [45].
Cell culture studies considering the OSS surface also compared it to its micrometer scale textured predecessor [38]. In this study, cell adhesion, proliferation, and alkaline phosphatase activity were assessed with human SaOS-2 17 osteoblasts and bone mesenchymal stem cells in nonosteogenic culture conditions. The results demonstrated higher osteoblastic differentiation for the nanometer scale surface relative to its micrometer scale counterpart [38].
In general, cell culture studies depicted favorable results for nanometer scale surfaces relative to their micrometer scale predecessors. To date, no such study has been performed for the DCD surface, and the mixed results observed for the IBAD surface relative to the uncoated AB/AE substrate were possibly related to the amorphous coating dissolution. Specific to the OSP and OSS surfaces, where similar micrometer-level textured surfaces were used as controls against nanometer scale within micrometer scale topography surfaces, it is unequivocal that the nanometer scale features substantially altered cell behavior favoring osteogenic cellular events.
Preclinical In Vivo Models
Unlike the mixed results obtained in cell culture assays for the IBAD surface, a series of studies demonstrated its superiority to uncoated surfaces in both biomechanical and histometric outcomes [46–48]. When cylindrical implants were utilized with the IBAD surface versus its uncoated counterpart, higher degrees of osteoconductivity were observed along with higher degrees of biomechanical fixation at early implantation times [46–48]. Furthermore, it has been demonstrated that the coating thickness played a role on biomechanical results [47]. When commercially available implants were utilized in larger preclinical in vivo models, significantly higher levels of bone-to-implant contact (BIC) and biomechanical fixation were observed [28, 33, 49–51]. While significant improvements were consistently obtained relative to its uncoated counterpart, studies that considered the IBAD- versus PSHA-coated implants demonstrated that the PSHA-coated implants outperformed the IBAD-coated groups, especially when biomechanical competence at early implantation time is concerned [33, 47, 50, 52].
The DCD surface showed promising results in rodent models relative to its micrometer scale textured counterpart [53–55]. In a bone healing chamber design in a rat model, higher degrees of bone ingrowth were observed [54], as well as higher degrees of bone adhesion were detected when bone pullout from DCD-coated implants was compared to the predecessor control (regarded as bone bonding due to the presence of nanometer scale features on the implant surface) [53]. However, different than observed in rodent models, no differences in bone response to both DCD and its predecessor surface in a beagle dog model were detected [56–59]. When the DCD surface was compared to other moderately rough surfaces in the challenging immediate extraction socket scenarios, it did present significantly lower BIC levels relative to a dual acid-etched surface, an SLA surface, and an anodized surface. When socket architecture was considered, no difference was detected between the four different implant groups [60].
The OSP surface has also been well documented in laboratory preclinical animal models versus its moderately rough micrometer scale textured predecessor and against other surfaces. Ellingsen et al. [61] demonstrated higher biomechanical competence and BIC levels for the OSP relative to its micrometer scale predecessor (TiOblast, Astra Tech AB, Mölndal, Sweden) in a rabbit tibia model. Through modification in the relationship between implant macrogeometry and surgical instrumentation (forming healing chambers between threads [6, 7, 24]), Berglundh et al. [34] demonstrated superior results for the OSP surface relative to its predecessor when the amount of bone formation within healing chambers was concerned.
The OSP surface has also been compared to other micrometer scale surfaces in numerous preclinical laboratory animal models. In a rabbit model, at 2 weeks in vivo, OSP surface presented significantly higher bone response when compared to an anodized surface presenting micrometer-level texturing [62]. In a crestal bone maintenance study conducted in minipigs by Heitz-Mayfield et al. [63], the OSP along with SLA implants presented higher degrees of crestal bone maintenance relative to an implant presenting an anodized surface with micrometer-level texturing [63].
The OSP has also been evaluated against other micrometer scale textured surfaces with enhanced surface wettability in fresh extraction sockets in beagle dogs [64], and after 4 and 12 weeks, no differences in host-to-implant response were detected. Another study comparing OSP versus other surfaces in fresh extraction sockets failed to demonstrate differences between groups in all parameters evaluated [65]. It should be noted that this particular study primarily comprised the evaluation of soft tissue measurement outcomes and not osseointegration measurements. However, bone maintenance around implants immediately placed in extraction sockets has been shown to influence soft tissue measurements [66, 67].
The OSP surface has also been compared to other nanometer scale surfaces in numerous preclinical laboratory animal models. These studies are described subsequently in the text.
The OSS has also been well documented versus its AB/AE predecessor in a series of preclinical in vivo studies. The first was conducted by Marin et al. [68], where OSS and AB/AE surfaces were histometrically and biomechanically evaluated in a beagle model. The group reported that while no significant differences were observed in BIC between surfaces at both 2 and 4 weeks, an approximately 100 % increase in removal torque was observed for the OSS surface relative to its predecessor counterpart, strongly suggesting that bone around the OSS surface presented higher mechanical properties [23]. Similar results were obtained by Marin et al. [69] when the OSS surface was compared to a dual acid-etched moderately rough surface presenting micrometer-level texture.
In a protocol similar to Mendes et al. [53], who reported bone bonding between the DCD nanometer scale modified surface and bone, Coelho et al. observed the same bone bonding phenomenon when the OSS surface was compared to its AB/AE micrometer scale textured counterpart, suggesting that bone bonding can also be achieved by the lower levels of Ca and P on the OSS implant surface (note that crystalline HA particles are present at much higher amounts for the DCD surface) [70]. Alternatively, the authors speculated that bone bonding to the OSS surface was possibly due to the nanometer scale texture rather than due to the low levels of Ca and P on the OSS surface relative to the DCD process. To address the question of whether nanometer scale texture or the surface chemistry was responsible for the high osteoconductive properties of the OSS surface, a nanometer scale presenting texture similar to the OSS surface was produced through silica blasting and thus no Ca and P content was present in its surface chemistry [71]. When these were compared in vivo in a beagle model, no differences in bone response (torque and BIC) were detected between surfaces, suggesting a stronger nano-texture contribution in the OSS surface osseointegration relative to its low level of Ca and P presence on its surface [71]. These results strongly suggest that the bone bonding achieved in Coelho et al. was likely due to the nanotopographical component of the OSS surface and experimental studies should be conducted to further address the nanometer scale texture and chemistry’s contribution to bone bonding to implant surfaces [70]. Another histometric, nanomechanical, and gene-expression study conducted in a rodent model unequivocally showed higher BIC, bone mechanical properties (hardness and modulus of elasticity), and osteogenic gene expression for the OSS surface versus its predecessor, indicating that the nanometer scale surface indeed modulates osteoblastic cell response, leading to faster osseointegration and bone mechanical property achievement [72]. Finally, the OSS surface when evaluated in a more challenging scenario such as immediate placement in extraction sockets was able to maintain higher levels of bone attachment at the buccal flange relative to implants presenting a smooth cervical region [73].
When compared to other micrometer scale surfaces, the OSS presented favorable biomechanical and histometric results. For instance, a study comparing the OSS surface relative to several other micrometer scale textured surfaces obtained through AB/AE and resorbable blasting media (RBM) alone, plasma-treated micrometer textured surfaces, and RBM acid-etched surfaces depicted significantly higher torque levels for the OSS surface relative to others [74]. Another study comprising the histometric and nanomechanical assessment of bone mechanical property of OSS versus OSP, SLA, anodized, and RBM surfaces demonstrated higher BIC for the OSS at the earliest time point in vivo and that bone mechanical properties slightly differed between surfaces but not to a significant extent [35].
Relative to other nanometer scale surfaces, the OSS surface was compared to the DCD surface and other micrometer scale textured surfaces in a canine model at 10 and 30 days postimplantation [29]. Worth noting is that in this study, all implant macrogeometries and surgical instrumentation were the same, minimizing osseointegration confounding factors due to implant hardware. This study showed that the OSS surface presented significantly higher biomechanical competence (assessed by removal torque) than the other groups at 30 days in vivo [29].
One study directly comparing the biomechanical performance of the OSS, OSP, and DCD implants is available in the literature. The implants were placed in the beagle dog mandible at 1 and 3 weeks in vivo prior to euthanasia. When torqued out, the OSS implant system presented significantly higher removal torque values compared to the OSP and DCD surfaces. At 3 weeks, both OSS and OSP implants presented similar results and both were significantly higher than the DCD surface [75]. While insightful, the results of this particular study must be interpreted with care, as it did not compare the performance of the three different surfaces but the performance of three different implant systems with nanometer scale surfaces. Specific to the removal torque results, it must be noted that the significant differences between groups may have been exacerbated by differences in implant hardware that possibly mitigated the effect of the nanometer scale in osseointegration.
Human Implant Retrieval Studies
Cell culturing and preclinical laboratory animal model studies are remarkable methods for initial evaluation of the effect of multiple scale design features in osseointegration. Even though slightly contradictory, the literature presented thus far regarding cell cultures and animal models in nanometer scale textured implants has shown the merit of such features in achieving favorable results in cell modulation, osseointegration, biomechanical fixation, and bone mechanical properties relative to micrometer scale surface texturing. While all laboratory preclinical work is valid to determine whether there is potential clinical application for novel design features, careful experimental work conducted in humans under appropriate IRB approval provides one of the most valuable tools for evaluation of both implant success and failures [76–78]. Once the removal of the implantable device is completed, this specimen contains important information regarding the biological reaction from the host and the effects of the implant presence in bone modeling/remodeling. Human implant retrieval studies have been conducted for some of the nanometer scale surfaces that have been made commercially available about the IBAD surface.
Orsini et al. [79] histologically and histomorphometrically evaluated the DCD surface versus its acid-etched predecessor in a study where both implant surfaces were placed in the posterior maxilla of 15 patients in a nested within subject study design. Following implant retrieval after 2 months healing, they evaluated the implants under a confocal microscope and concluded that the DCD presented significantly higher degrees of osseointegration relative to its predecessor [79].
Rocci et al. [80] compared OSP implants versus its micrometer scale textured predecessor placed in the mandible in a nested within subject design. Eight weeks after placement, the implants were retrieved and histometrically evaluated. Despite the small number of samples, the overall results of all histometric outcomes favored OSP relative to its control. In a study that evaluated the early temporal-wide genome transcription regulation by the surface topography at the bone–implant interface between OSP and its predecessor, paired samples collected at 3 and 7 days after placement from 11 healthy patients demonstrated that collagen fibrillogenesis, extracellular matrix organization, and the inflammatory/immune responses were observed in implant adherent cells early (3–7 days) after implantation [81]. The same study also reported different gene expression between surfaces at 3 and 7 days, further reinforcing that surface modifications do have strong potential for modulating cell behavior [81].
The OSS surface was also evaluated against its predecessor in a study where implants were placed in pairs in the posterior maxilla of ten subjects [82]. Implants were retrieved following a period of 8 weeks. The histometric results showed significantly higher BIC and osteocyte density within the newly formed bone for the OSS versus the control [82].
Clinical Research
Outcomes of a few published clinical trials regarding some implant surfaces with nanotopography will be described in this section and summarized in Table 5.1. The DCD surface has been evaluated in a prospective 1-year clinical study with tapered implants placed in 42 patients, with 55 % located in the posterior region (20 single-tooth, 30 fixed dental prostheses, and 7 full-arch maxillary reconstructions). Survival rate at 1 year was 99.4 % [83]. The same surface was evaluated in an immediate-loading prospective 1-year clinical study but on a different macrogeometry (Prevail®). Thirty-five patients received 102 implants (65 % in the posterior region), to support 14 single crowns (SC), 26 fixed dental prostheses (FDP), and 4 full-arch reconstructions. The survival rate was 99.2 % (one implant failure) [84].
Table 5.1
Clinical outcomes of prospective studies of implant surfaces presenting nanotopography
|
Author/implant surface
|
Prostheses design
|
Survival rate (%)
|
Observation period (study design)
|
Immediate occlusal loading
|
Control group (non nano-enabled surface)
|
|---|---|---|---|---|---|
|
Östman et al. [83]/DCD
|
20 SC, 30 FDP, 7 FA
|
99.4 % (one implant failure)
|
1 year (prospective)
|
Yes
|
No
|
|
Östman et al. [84]/DCD
|
14 SC, 26 FDP, 4 FA
|
99.2 % (one implant failure)
|
1 year (prospective)
|
Yes
|
No
|
|
Cecchinato et al. [85]/OSP
|
91 SC
|
Not described
|
3 years (prospective)
|
Yes
|
No
|
|
Collaert et al. [86]/OSP
|
25 FA
|
100 %
|
2 years (prospective)
|
Yes
|
No
|
|
De Bruyn et al. [87]/OSP
|
132 SC
|
94–98 %
|
3 years (prospective)
|
No
|
No
|
|
Mertens and Steveling [88]/OSP
|
31 SC, 4 FDP, 1 FA
|
97 %
|
5 years (prospective)
|
Yes/early loading included
|
No
|
|
Mertens et al. [89]/OSP
|
Fixed and removable
|
97.85 %
|
28 months (prospective)
|
No
|
No
|
|
Noelken et al. [90]/OSP
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


