Fig. 11.1
Clinical situations with compromised alveolar bone sites
During the last decades great interest has been shown for biofilm formation on exposed implant surfaces and its sequelae [7]. Especially, the influence of the surface characteristics for developing peri-implantitis has been examined in animal studies, where significant differences between implant surface modifications have been found [8]. Thus, critical clinical situations arise when implant surface coatings originally designed to accelerate and improve bone healing become exposed to oral biofilm and increase the risk for peri-implant inflammation and bone destruction (Fig. 11.1).
Macro-, Micro-, and Nanocoatings
Basically, surface modification can be performed by subtractive techniques, e.g., acid etching, oxidation, and blasting procedures, resulting in a plastic deformation of the surface, or by additive techniques, resulting in depositions of the surface. The latter is called a coating, and this may affect chemical inertness, surface energy, hydrophilicity, cell adhesion, and resistance to infections. Coatings can have different thickness and roughness. A few decades ago coatings within orthopedics and implant dentistry were macro- and microcoatings, e.g., titanium plasma-spray coatings and hydroxyapatite coatings, with coating thicknesses 30 μm and upwards [9]. This period was succeeded with implant surface modifications using a microscale surface topography, described with a great number of surface characterizing height, space, and hybrid parameters with the average height deviation from a mean plane (Sa-value) as the most frequently describing surface roughness parameter. Thus, surfaces were divided into smooth (Sa = 0.0–0.4 μm), minimally rough (Sa = 0.5–1.0 μm), moderately rough (Sa = 1.0–2.0 μm), and rough (Sa >2 μm). The change from smooth to moderately rough implant surfaces improved the clinical outcome of implant therapy significantly [4]. Today most dental implants have moderately rough implant surfaces, and an increasing number of companies add nanocoatings, where the thickness of the coating is measured on a nanoscale (1–100 nm). In contrast to the moderately rough implant surface modifications, no clear evidence exists that nanocoatings will improve the clinical outcome of implant therapy. This will also be difficult as the 5- and 10-year survival rates of implants with moderately rough surface are reported to be better than 97 % [4, 10]. Thus, nanocoatings or new surface modifications will primary be indicated for compromised bone sites or cases with a need for accelerated bone healing.
Most nanocoatings are characterized as bioactive. This has been defined as a surface which elicits biological activity on the surrounding tissue [11]. A biochemical bonding between implant surfaces and surrounding tissue is aimed in contrast to only microstructured surfaces, where the interface results in a physical bonding depending on the surface topography at the micrometer level. It is, however, important to realize that by changing one surface property other surface properties, chemical and physical, will consequently be affected. The chemical strategies with nanocoatings focus on interface bonding using inorganic and/or organic molecules [6] (Table 11.1).
Table 11.1
Examples of nanocoatings used for dental implants
|
Chemical group
|
Chemical subgroup
|
Substance/molecule/fluid
|
References
|
|---|---|---|---|
|
Inorganic
|
Element (periodic table)
|
Diamond
|
[12]
|
|
Fluoride
|
[13]
|
||
|
Silver
|
[14]
|
||
|
Calcium phosphate
|
|||
|
Zinc
|
[14]
|
||
|
Titanium
|
[17]
|
||
|
Organic
|
Protein
|
Collagen I
|
[18]
|
|
Elastin
|
[6]
|
||
|
Fibronectin
|
[19]
|
||
|
Laminin
|
[20]
|
||
|
Osteopontin
|
[21]
|
||
|
Bone sialoprotein
|
[22]
|
||
|
Growth factors
|
BMP-2, BMP-4, BMP-7
|
||
|
Peptides
|
RGD
|
[24]
|
|
|
Parathyroid hormone
|
[25]
|
||
|
Antimicrobial Gl13K
|
[26]
|
||
|
Polysaccharides
|
Hyaluronic acid
|
[27]
|
|
|
Chondroitin 4-sulfate
|
[28]
|
||
|
Chitosan
|
[27]
|
||
|
Pectins
|
[6]
|
||
|
Drugs
|
Bisphosphonate
|
[29]
|
|
|
Simvastatin
|
[30]
|
||
|
Strontium ranelate
|
[31]
|
Inorganic Coatings
Titanium plasma coating is one of the most well-known surface modifications used in implant dentistry. It was adopted from the orthopedic field and had a coating thickness between 30 and 40 μm. An arc flame temperature of 15–20,000 °C and a gas jet velocity more than 3,000 m/s characterized the coating technique called titanium plasma spraying (TPS). The titanium powder grain size was 0.05–0.1 mm and resulted in a very rough implant surface [9]. It could be characterized as a porous surface with approximately ten times greater surface area than the titanium surface without coating [17]. Experimental studies with the TPS surface demonstrated osseointegration, and clinical studies reported good survival rates even in clinical cases with low bone quantity [32]. However, peri-implant infections adjacent to the biofilm exposed implant surface were also reported [33] and abandoned the surface coating.
Coating of medical implants with calcium phosphates (CaP) including hydroxyapatite (HA) has been done for several decades to increase the biocompatibility of the implant and enhance peri-implant bone formation [15]. Originally, the HA coatings were plasma sprayed by high temperature at the implant surface, resulting in a very thick and rough coating. In clinical reality adhesion failure and cracking were reported on the thick HA coatings [34] and in dentistry also peri-implantitis. By using new physical deposition and wet-chemical techniques, very thin CaP coatings <100 nm have been developed, and the requirements to HA coatings have been described in details [14]. Numerous studies have been performed with CaP nanocoatings, and most in vitro experiments have shown an enhanced osteoblast-like cell adhesion, proliferation, and differentiation indicating an accelerated and increased bone formation [14]. CaP coatings have frequently been used as carrier for organic molecules. Various bioactive molecules that promote bone regeneration in vitro have been incorporated into CaP coatings [14]. The adhesion of bioactive molecules or drugs at implant surfaces may modulate integrin binding and subsequent cellular response (Fig. 11.2). Thus, several examples of proteins, e.g., BMP-2, albumin, and amelogenin, incorporated into the latticework of CaP have been described, and CaP implants have been immersed into solutions with antimicrobial agents [35]. Although exciting theories for osteoconduction of CaP nanocoatings have been described and a vast number of promising in vitro and animal studies exist, the exact mechanism behind the biological response of CaP coatings is still unclear. It has also been reported that CaP nanocoating does not favor in vitro osteogenesis [36], and a recent well-controlled in vivo study in dogs could not either confirm that CaP nanocoatings improve early bone integration [37].
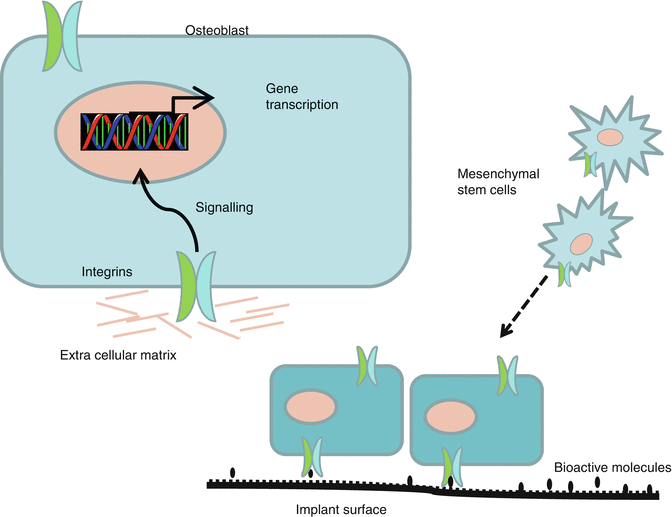

Fig. 11.2
Cells with targeting integrins to promote bone formation at implant surfaces coated with bioactive molecules
An attractive issue is cosubstitution of CaPs with metal ions, which are present as trace elements in bone. Several ions such as fluoride, strontium, zinc, silver, and even nanoparticle diamond [12] have exerted stimulatory effects on osteoblast-like cell activities in vitro both as additive to CaPs and as solely nanocoatings [14]. From a clinical point of view, it is interesting that an antibacterial effect of several metals, e.g., zinc and silver ions, has been reported [14]. However, no in vivo studies have until now examined the impact of the antibacterial effect on implant outcome.
Fluoride has been analyzed in several in vitro and in vivo studies described in a former chapter of this book, and the research has been translated to a dental implant system with success, although no randomized controlled trials (RCT) have demonstrated better outcome with fluoride-coated implant than other titanium implants [38]. The studies comparing fluoride-coated implants with non-fluoride-coated implants suffer from the fact that the surface topography of the test and control implants was unequal. Thus, the positive influence reported on fluoride-coated surfaces may be more related to the changed surface topography than the chemical effect of the fluoride.
Organic Coatings
Proteins mainly represent organic nanocoatings, but also glycoproteins and polysaccharides have obtained increased attention as bioactive molecules. Surface modifications with carbohydrates are easy to obtain and inexpensive and can be tailored with many compositions and structures. Osteoblasts with integrins recognize and adhere directly or indirectly through adhesive proteins to surfaces coated with polysaccharides, and a number of studies have indicated that different polysaccharides especially pectins are able to increase the hydrophilicity of surfaces and enhance the mineralized matrix formation of osteoblastic cells [6]. In vivo studies confirming the in vitro reports are, however, still not published, and although pectins have a number of advantages as nanocoatings compared to proteins, e.g., persist at the surface for longer time, with antibacterial effect, and much cheaper, the ideal tailored polysaccharide for bone implants still remains to be developed.
The potential of bone proteins as biomimetic agents have been well documented in cell cultures and animal models. Bone morphogenic proteins (BMPs) have shown significant enhancement of osseointegration [23], but especially the high cost of BMPs and the release profiles have limited their clinical use. Fibronectin, laminin, osteopontin, bone sialoprotein, elastin, and collagen as well as RGD peptides are other proteins used for biochemical modification of dental implant surfaces [6]. The cells are bonded to the proteins through different integrin receptors in the cell membrane (Fig. 11.2). Although studies have demonstrated positive effects of several proteins, a great number of challenges exist – concentration to be used, longevity of the coating, and the serious adverse effects caused by increased cellular activity. The risk of angioma formation following uncontrolled delivery of vascular endothelial growth factor has been described [39]. Adverse effects have to be properly examined before bioactive growth factors and biomimetic agents can be clinically applied.
From an academic point of view, it is also interesting that drugs as bisphosphonates (BPs), strontium ranelate, hormone therapy, and monoclonal antibodies that bind RANKL used in the treatment of osteoporosis and other bone diseases will cause increased bone density and mineralized bone-to-implant contact [40]. Most medical therapies for osteoporosis primarily inhibit bone resorption and reduce bone remodeling. However, parathyroid hormone has the potential to enhance skeletal microarchitecture [25]. Nevertheless, agents influencing the balance between bone resorption and formation can result in a higher bone density. The antiresorptive agents also illustrate that a high bone density or bone-to-implant contact is not equal to successful clinical outcome as a number of case reports for patient treated with high doses of bisphosphonates have caused osteonecrosis of the jaw after surgical procedures [41].
Relation Between Methods and Clinical Reality
When new implant surface coatings are introduced, a great variety of methods are used to document the efficacy of the coating. Before clinical human studies are initiated, biomechanical, histological, microradiographic, and molecular analyses are performed in controlled studies using coated implants, plates, or disks.
Biomechanical tests are used to evaluate the strength of the attachment between bone and implant surface. Push out and removal torque tests have been used intensively in animal studies to examine shear forces, but also pull out test for measuring tensile forces is a measure for attachment strength [42]. Biomechanical tests are important from a clinical point of view, but are quite coarse and mainly depending on the surface topography at the micrometer level. Thus, when bioactive surface modifications are tested, the surface topography should not be changed at the micrometer scale, as this would imply a risk for misinterpretation of the chemical effect. Studies with nanocoatings have also frequently indicated that the sensitivity of biomechanical tests of nanoscale surface changes is low [43] and other analyses, e.g., molecular analysis, may be more sensitive. On the other hand it can be argued that if standardized biomechanical and histological tests don’t show significant benefits of a new surface modification, it has little clinical relevance.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


