7 Evaluation of Salivary Glands—Imaging
Indications, Advantages, and Disadvantages
Technique, Advantages, and Disadvantages
Technique, Advantages, and Disadvantages
Introduction
Patients with major salivary gland lesions may present to the otorhinolaryngologist, oral surgeon, or maxillofacial surgeon with a variety of symptoms such as pain, swelling, drooling, facial nerve palsy, or erythema of the overlying skin. Many diseases may not require any imaging at all, as they can be evaluated with palpation and with endoscopic or transoral direct visualization. However, other entities such as malignant tumors may require a thorough preoperative assessment with high-resolution magnetic resonance imaging (MRI) or with contrast-enhanced positron-emission tomography (PET) or computed tomography (CT).
The algorithm for imaging the salivary glands varies depending on the history with which the patient presents, on the clinical findings, and on institutional preferences. These may also differ between continents—for example, ultrasound is rarely used in North America to assess calculi or superficial salivary gland lesions, whereas the technique is very popular in Europe and Asia and may replace CT or MRI if performed by an experienced operator in a given clinical context. A wide range of studies are needed to assess salivary gland lesions, including conventional radiography, conventional sialography, radionuclide scanning, ultrasound, CT, MRI, MR sialography, and PET/CT.1–4 In this chapter, each imaging technique is reviewed separately and the major indications are discussed.
Conventional Radiography
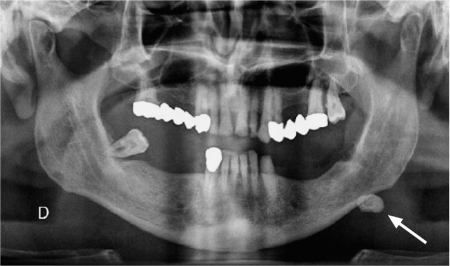
Conventional radiographs include oblique lateral films of the mandible, intraoral occlusal views, anteroposterior views of the parotid region, and panoramic radiography. Although standard radiographic views were obtained routinely to detect salivary gland calculi in the past, they have nowadays been replaced by a variety of cross-sectional imaging techniques. Conventional radiography is the least sensitive technique for detecting calculi. As only 60%–80% of salivary gland calculi are radiopaque, conventional radiography may miss up to 20%–40% of calculi.1,2 In addition, a variety of preexisting calcifications, such as phleboliths, tonsilloliths, and lymph-node calcifications may be mistaken for calculi on conventional radiographs. However, salivary gland calculi may be detected incidentally on conventional radiographs, typically in panoramic views, and they have to be correctly identified and reported as such (Fig. 7.1).
 Conventional radiography is not very sensitive for detecting calculi; 20%–40% of the stones are not detected.
Conventional radiography is not very sensitive for detecting calculi; 20%–40% of the stones are not detected.
Fig. 7.1 As an incidental finding in a patient with suspected dental infection, this panoramic view shows a large calcification on the left (arrow), compatible with a submandibular calculus. Ultrasound confirmed a large calculus in the Wharton duct.
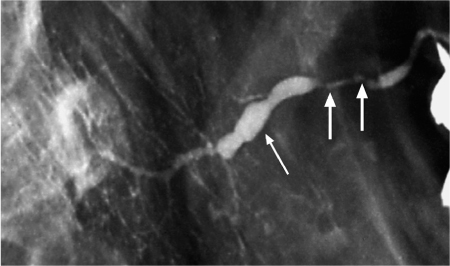
Fig. 7.2 Chronic sialadenitis of the parotid gland, as seen on conventional sialography. There is sialodochitis involving the Stensen duct, with areas of stenosis (arrows) and dilation (thin arrow). The irregular caliber of the intraglandular branches and scattered collections of contrast medium, which represent small intraglandular abscesses, should be noted.
Conventional Sialography
 Indications
Indications
Before the advent of CT and MRI, conventional sialography, particularly digital subtraction sialography, used to be the mainstay for diagnosing inflammatory and neoplastic lesions of the salivary glands.1–3,5,6 Its use for assessing salivary gland masses has now been abandoned, but the technique is currently still considered to be the most powerful imaging modality for assessing the presence or absence of ductal abnormalities (Fig. 7.2), including calculi, and for evaluating patients with sialosis, although it is increasingly being replaced by ultrasound and MR sialography. In some institutions, including our own, MR sialography has completely replaced conventional sialography for the assessment of ductal disease, Sjögren syndrome, and recurrent parotitis in children (see below). Although conventional sialography can depict the parotid and submandibular ductal system in great detail, the technique is not capable of detecting pathology in the sublingual glands unless patients present with anatomic variations, such as filling of the Bartholin duct when the Wharton duct is injected during submandibular sialography.
 Conventional sialography is still widely used to detect ductal pathology, mainly for ductal stenoses and salivary stones. In many institutions, however, it has been replaced by ultrasound and MR sialography.
Conventional sialography is still widely used to detect ductal pathology, mainly for ductal stenoses and salivary stones. In many institutions, however, it has been replaced by ultrasound and MR sialography.
 Technique
Technique
Conventional sialography is performed with standard fluoroscopic equipment. Before the start of the examination, conventional radiographs are obtained in anteroposterior and lateral oblique projections to detect grossly radiopaque sialoliths. For optimal visualization of the intraoral opening of either the Stensen or Wharton duct, all patients receive a secretogogue (fresh lemon) before the examination starts. The standard sialographic equipment includes lacrimal dilators, sialographic cannulas, a polyethylene connecting tube, a 5-mL syringe, and a lowosmolarity water-soluble contrast agent such as ioxaglate sodium or ioxaglate meglumine. Cannulation can be done with the patient seated or supine.
The parotid duct orifice is usually easily identified in the buccal mucosa opposite the upper second molar tooth. As the orifice of the submandibular duct is smaller than that of the parotid, magnifying spectacles are often needed to identify its opening in the sublingual caruncle, lateral to the frenulum of the tongue. Once the ductal opening has been identified, the dilators are used to widen it for easier cannula placement. The cannula is advanced gently to avoid perforation, and contrast is injected slowly using manual pressure. The injection is always performed under fluoroscopic control to achieve optimum ductal filling, and spot radiographs are subsequently obtained in the anteroposterior and lateral–oblique projections to document the examination.
A radiograph is obtained on evacuation to document ductal emptying after repeat administration of fresh lemon. Thispost-evacuation film makesit possibletodetectextravasation ofcontrast, and itcan be used to evaluate the pattern of emptying. Delayed contrast evacuation may be due to inflammation, obstruction, or processes associated with parenchymal destruction, such as Sjögren disease, sialosis, chronic infection, and radiation-induced sialadenitis.
 Contraindications
Contraindications
Acute salivary infection is an absolute contraindication to conventional sialography.5,6 Sialography should also not be performed in patients with known allergy to iodinated contrast media, as fatal anaphylactic reactions have been reported in the literature. In patients scheduled for thyroid function studies, sialography should be postponed until the thyroid work-up is completed.
 Advantages and Disadvantages
Advantages and Disadvantages
The advantages of conventional sialography include multi-planar high-resolution imaging of the salivary ducts, including third-level and fourth-level branches, and assessment of ductal function in response to a sialagogue; therapeutic release of stones is also sometimes possible after retrograde injection of contrast. Its disadvantages include radiation exposure, dependence on the operator’s technical skills for successful cannulation, and the need for retrograde injection of contrast. Contrast injection may also result in displacement of an anterior ductal stone into a posterior position where it can no longer be reached by an intraoral surgical approach, or where endoscopic removal is more difficult.
Potential complications of conventional sialography include damage to the orifice, overfilling and rupture of the ductal system, exacerbation of infection, and adverse reactions to contrast media. Failure to cannulate the ductal orifice may occur if there is calculus at the ductal orifice, with related edema of the surrounding buccal mucosa, or in the absence of any ductal abnormalities. Smaller calculi and subtle ductal abnormalities near the ductal orifice may be obscured by the injection of contrast, and overfilling may result in a false-positive diagnosis of sialectasis. Rarely, air bubbles may be mistaken for calculi, leading to false-positive assessments for lithiasis.
Radionuclide Scanning
 Examination Technique
Examination Technique
The principle for imaging the salivary glands with technetium Tc 99 m pertechnetate is based on the ability of the intercalated duct epithelial cells in the salivary glands to transport large monovalent anions, such as iodide and pertechnetate, from the surrounding capillaries and to secrete them into the saliva. Radioisotope scanning with 99mTc pertechnetate provides functional information about trapping and excretion in the parotid and submandibular glands.1,7 The technique does not require any specific patient preparation. However, thyroid-blocking agents are not allowed within 48 hours of the examination. The examination is conducted with a gamma camera.
After intravenous injection of 150–450 mBq of99mTc pertechnetate, both dynamic and static images of the major salivary glands are acquired. The examination field includes the thyroid gland, which is used for reference purposes. The dynamic and static images are first obtained with the patient in the Waters view position, after which lateral and oblique lateral static images are acquired. After completion of the static images, a sialogogue (lemon juice) is administered, and the washout images are registered 5–10 minutes after the stimulant.
A normal scan typically shows a distinct, symmetrical concentration of activity in the parotid, submandibular, and thyroid glands 1 minute after intravenous injection of99mTc pertechnetate. Within 5–10 minutes, the levels of activity increase in the parotid and submandibular glands. After 15 minutes, there is a significant decrease in activity due to spontaneous excretion into the mouth. The washout images demonstrate salivary gland excretion, which is complete 5–10 minutes later. Generalized salivary gland dysfunction, as seen in Sjögren syndrome, results in decreased uptake of99mTc pertechnetate in comparison with the thyroid as the reference level.
 As the thyroid gland is used for reference purposes, thyroid gland dysfunction may lead to inadequate functional assessment of the salivary glands.
As the thyroid gland is used for reference purposes, thyroid gland dysfunction may lead to inadequate functional assessment of the salivary glands.
 Current Indications
Current Indications
Radionuclide scans are rarely used today, as they are not as accurate as ultrasound, CT, or MR for demonstrating lesions in the salivary glands. In the past, radioisotope scanning with99mTc pertechnetate was useful for establishing a diagnosis of Warthin tumors and oncocytomas, as these tumors regularly show increased uptake of the isotope and appear as “hot spots” on the radionuclide scans. The main indication today is for evaluating the functional status of the major salivary glands in patients with normal ultrasound, CT, or MRI examinations. The technique may help identify subclinical involvement of the salivary glands in systemic diseases such as sarcoidosis, connective-tissue disorders, or Sjögren syndrome.1–3,7
After initially demonstrating increased trapping in the acute phase, glands with systemic disease involvement tend to show decreased trapping and failure to discharge pertechnetate in response to an appropriate physiologic stimulus. It has recently been shown that patients with Sjögren syndrome who present with severe scintigraphic involvement at diagnosis appear to have more pronounced autoimmune expression, a higher risk of developing systemic features and lymphoma, and a lower survival rate. It has therefore been suggested that the degree of salivary gland dysfunction assessed by parotid scintigraphy may offer valuable clinical information on the prognosis and outcome in patients with primary Sjögren syndrome.7
 The main indication for radionuclide scanning today is for evaluation of the functional status of the major salivary glands in the presence of normal ultrasound, CT, or MRI examinations, or in clinical studies in which the method is used to quantify salivary function.
The main indication for radionuclide scanning today is for evaluation of the functional status of the major salivary glands in the presence of normal ultrasound, CT, or MRI examinations, or in clinical studies in which the method is used to quantify salivary function.
Ultrasonography
 Examination Technique
Examination Technique
Ultrasonography is a quick, noninvasive, and relatively inexpensive method of assessing salivary gland pathology that involves no radiation exposure. Ultrasonography is best performed using linear-array broadband transducers with a frequency of ≈ 7–12 MHz. As in other parts of the body, selection of the transducer frequency depends on the depth of the lesion to be examined and on the attenuation of the interposed tissues. To examine very superficial lesions, a standoff pad made of silicone elastomer can be used in selected cases. In large lesions, additional transducers with a lower frequency are advisable in order to delineate the lesion completely. Although color Doppler and power Doppler imaging are often very useful for salivary gland examinations, most diagnoses are made using the standard gray-scale technique. Bilateral investigation of the salivary glands is extremely important, as many diseases occur bilaterally.
 Indications, Advantages, and Disadvantages
Indications, Advantages, and Disadvantages
Ultrasonography of the salivary glands is currently used to evaluate calculi, inflammatory diseases, and congenital lesions, and in combination with fine-needle aspiration cytology it is a powerful tool for characterizing salivary gland tumors.8–10 It is helpful for assessing the vascularity of the gland and in deciding whether a mass lesion is solid or cystic.
However, ultrasound is operator-dependent and does not allow complete cross-sectional imaging of the salivary glands. The deepest portion of the submandibular gland may be difficult to visualize, and assessment of the deep lobe of the parotid gland may be impossible due to the adjacent mandible. In addition, ultrasound does not demonstrate the intraglandular facial nerve branches and does not allow assessment of the skull base or mandible in infiltrative lesions.
Sialolithiasis
Although the indications for ultrasound examinations of the salivary glands may vary considerably from one institution to another, most investigators agree that, in experienced hands, ultrasound can be used as a first-line examination to detect sialolithiasis.1–3,8–10 The reported sensitivity of ultrasound for detecting salivary gland calculi varies between 71% and 94%, and the specificity may vary between 80% and 97%. Ultrasound is capable of detecting radiolucent calculi that have been missed on conventional radiographs. Familiarity with the typical sites in which calculi may be found increases the diagnostic yield. In the submandibular gland, typical locations for calculi are the genu of the Wharton duct and the confluence of the intraglandular ducts, whereas sialoliths in the parotid gland are often located in the peripheral parenchyma.
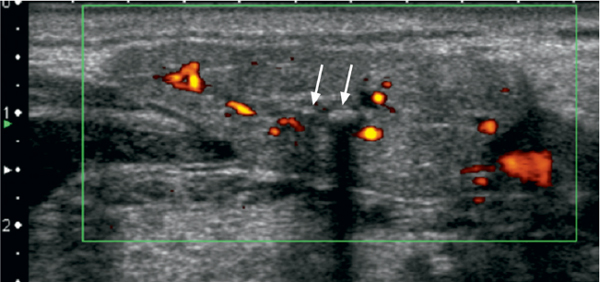
Sonographically, calculi have a typical appearance as bright curvilinear echo structures with a posterior acoustic shadow (Fig. 7.3). However, calculi smaller than 2 mm may not always display this characteristic posterior shadowing. As suggested by some authors, the lack of a dorsal acoustic shadow may depend not only on the size, but also on the chemical composition of calculi. Although there is often concomitant ductal dilation in symptomatic sialolithiasis, a lack of ductal dilation does not exclude the presence of lithiasis. Sialadenitis caused by ductal obstruction is seen in as many as 50% of salivary glands with calculi and may present with diffuse hypoechogenicity of the gland and characteristic hypervascularization on color Doppler images (Fig. 7.3).
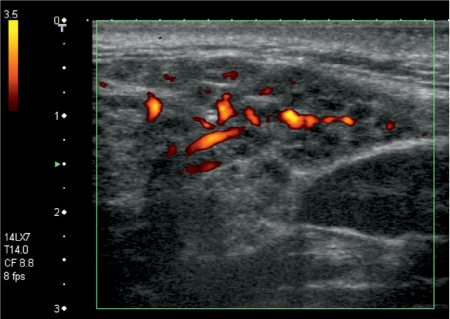
Fig. 7.3 Sialolithiasis and sialadenitis, as seen on color Doppler ultrasound of the submandibular gland. Two small calculi, 2 and 3 mm in size, respectively (arrows), are seen as hyperechoic structures with posterior shadowing. The increased vascularization of the slightly enlarged gland should be noted.
Sialadenitis
In acute sialadenitis, the main role of ultrasonography is to exclude ductal obstruction by calculi and detect intraglandular or periglandular abscess formation. Depending on the clinical situation, ultrasound may not be sufficient, and the imaging work-up has to be completed with CT or MRI (Fig. 7.4). Sialadenitis presents sonographically with enlarged glands, which have a characteristic rounded shape and an inhomogeneous, hypoechogenic structure. Color Doppler typically demonstrates hyperemia.
In chronic sialadenitis, the sonographic changes are often more subtle than in acute sialadenitis, and ultrasound is less accurate than in acute inflammatory disease. The echogenicity, shape, and vascularization of the gland may vary considerably. In addition, stenoses, which are often the cause of obstruction, cannot be assessed with ultrasound. In this clinical situation, MR sialography is indicated to allow precise assessment of ductal abnormalities (see below).
Other Causes of Chronic Inflammation of Salivary Glands
Other causes of chronic inflammation include radiation therapy, Sjögren syndrome, and granulomatous diseases such as tuberculosis and sarcoidosis. In the chronic fibrotic form of radiation-induced sialadenitis, the vascularization of the gland is reduced, the echo structure is inhomogeneous, and the size of the glands tends to decline over time. In Sjögren syndrome, the sonographic changes correlate with the histologic findings, and ultrasound may depict characteristic small cystic areas within the major salivary glands (Fig. 7.5). However, these findings are seen later in the disease process than with conventional sialography or MR sialography.
Tumors
Sonography can be used to differentiate between intrinsic and extrinsic masses, between cystic and solid lesions, and to assess superficially located tumors of the salivary glands.8,9 In most institutions, however, sonographic assessment is completed with fine-needle aspiration cytology (see Chapter 8) even if the ultrasound features may suggest a benign tumor, because malignant tumors such as adenoid cystic carcinoma or mucoepidermoid carcinoma may mimic benign tumors (see Chapter 29). As ultrasound is not capable of detecting perineural spread or skull base involvement in malignant tumors, high-resolution MR imaging is usually performed preoperatively (see Chapter 29). Owing to their high acoustic impedance, most salivary gland tumors are easily detected with ultrasound in normal echogenic salivary glands. However, the presence of inflammatory findings with diffuse pathologic parenchymal changes may make it impossible to detect small lesions.
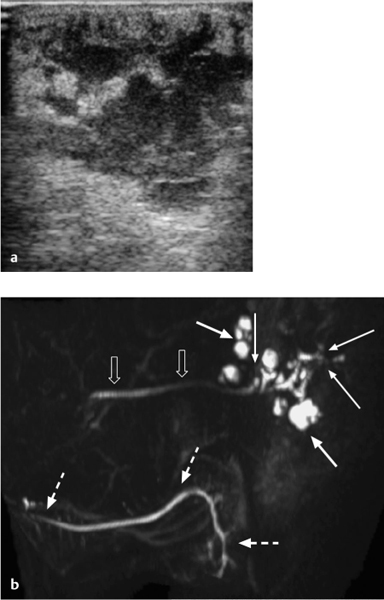
Fig. 7.4 a, b Recurrent sialadenitis of the parotid gland: ultrasound and magnetic resonance sialography findings.
a The ultrasound examination shows an enlarged parotid gland, with multiple poorly defined hypoechoic areas. No calculi were seen. Assessment of the ductal system was not possible with ultrasound alone.
b The magnetic resonance sialography examination (sagittal maximum-intensity projection) performed the same day shows multiple areas of ductal dilation with intraglandular sialoceles (arrows), stenoses of the peripheral ducts (thin arrows), and a normal-appearing Stensen duct (block arrows). The normal-appearing ipsilateral submandibular ductal system (dashed arrows) should be noted.
Fig. 7.5 Advanced Sjögren syndrome, as seen on color Doppler ultrasound of the parotid gland. The characteristic hypoechoic, cystic areas scattered throughout the gland and increased vascularization should be noted. Both parotid glands were involved.
 Ultrasound is a quick, noninvasive, and inexpensive method that does not involve any radiation exposure. If it is available, ultrasound is the first-choice imaging technique for assessing the salivary glands (see also Chapters 5 and 6).
Ultrasound is a quick, noninvasive, and inexpensive method that does not involve any radiation exposure. If it is available, ultrasound is the first-choice imaging technique for assessing the salivary glands (see also Chapters 5 and 6).
< div class='tao-gold-member'>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses