Fig. 1.1.
Intra-crystallite resin encapsulation of central hole regions of partially dissolved apatites (arrows) in bonded enamel (unstained, undemineralized TEM)
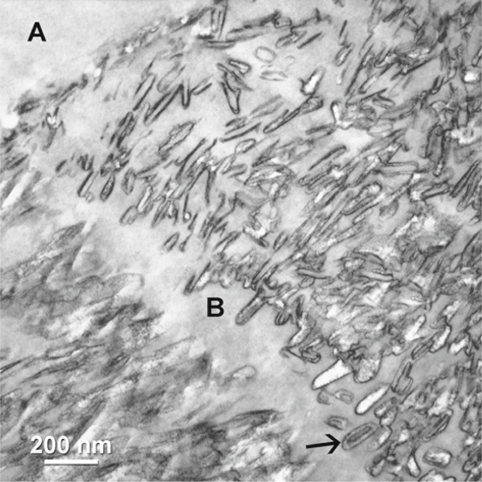
Fig. 1.2.
Adhesive (A) infiltration into interprismatic boundaries (B) within enamel hybrid layer. Arrow indicates central hole region of a partially dissolved crystallites (stained, demineralized TEM).
With the advent of contemporary dentine adhesives that contain hydrophilic resin monomers to enhance their coupling with wet dentine substrates, there was a paradigm change by applying these adhesives simultaneously to enamel and dentine [31, 32]. Two main strategies are currently in use for bonding to enamel and dentine: the total-etch technique and the self-etch technique [33].These adhesives are currently available as three-step, two-step and single-step systems depending on how the three cardinal steps of etching, priming and bonding to tooth substrates are accomplished or simplified [34]. Two-step systems are subdivided into the single bottle, self-priming adhesives that require a separate etching step and the self-etching primers that require an additional bonding step. The recently introduced single-step, self-etch adhesives further simplify bonding procedures into a single-step application. Both the two-step self-etching primers and the single-step (all-in-one) self-etch adhesives contain increased concentration of ionic resin monomers with acidic phosphate or carboxylic functional groups, rendering them aggressive enough to etch through the smear-layer-covered, cut dentine [35] and enamel [36]. Despite a less pronounced enamel-etching pattern, a similar retention mechanism via nanoretention of the partially dissolved apatite crystallites with resin has been observed with the use of self-etch adhesives on enamel [37]. Irrespective of how they are packaged, single-step self-etch adhesives are supplied as two-component assemblies, separating the functional acidic monomers that are liable to hydrolytic degradation from the water component which must be present to effectuate hard-tissue demineralization in order to maintain adequate shelf lives. They are mixed together immediately before use, and the mixture of hydrophilic and hydrophobic resin components is then applied to the tooth substrate. Some of the commercially available single-step, self-etch adhesives are disguised as “single-bottles” by hiding the catalysts in a sponge (AQ Bond/Touch & Bond, Sun Medical, Shiga, Japan; Parkell, Farmington, N.Y.) or applicator tip (AQ Bond Plus/Brush&Bond, Sun Medical Inc./Parkell) which must be used for activating the adhesive [38]. No-mix, single-step self-etch adhesive is also becoming available (iBond, Heraeus Kulzer, Hanau, Germany) that can accomplish etching, priming and bonding simultaneously to enamel and dentine immediately after dispensing [39].
The Enamel Smear Layer: A Potential Problem in Bonding to Cut Enamel
During the early stages of enamel bonding, few researchers understood that burcut tooth surfaces were covered by the smear layer [40, 41]. These adherent layers of cutting debris masked the underlying prismatic enamel and could not be rinsed off with water (Fig. 1.3). Resins applied to smear-layer-covered surfaces bonded to the relatively weak smear layers, rather than to the underlying hard tissues. The early adhesives were relatively hydrophobic and could not penetrate these smear layers. When the bonds were stressed to failure at approximately 5 MPa, examination of both sides of the failed bonds revealed that they were covered with smear-layer material, i. e. the apparent bond strength of 5 MPa was actually a measure of the cohesive strength of smear layers [42]. Minimal or no adhesive penetration in enamel surfaces could be identified even when contemporary hydrophilic singlebottle adhesives were employed in the absence of phosphoric acid etching [43].
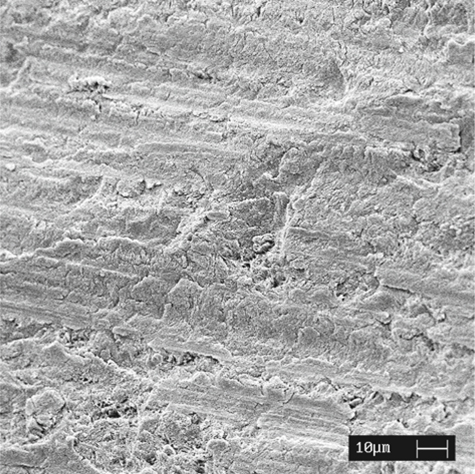
Fig. 1.3.
An SEM image of the enamel smear layer after cutting enamel with a diamond bur
Morphological studies on smear layers have focused primarily on the dentine smear layer, with the intention of preserving or modifying it with dentine-adhesive primers, as these studies were performed during a period when it was considered a taboo to place acid directly on dentine. The preservation of the smear layers and the smear plugs within dentinal tubules was considered beneficial in reducing the hydraulic conductance in bur-cut dentine [44, 45], as dentine permeability increased rapidly during acid-etching with even 6% citric acid, reaching a maximum value as early as 15 s of etching [46]. The inclusion of bacteria in dentine smear layers also generated concerns about their rapid propagation following dissolution of the smear plugs by oral fluids that could result in their colonization within the dentinal tubules and subsequent pulpal infection [47].
Although enamel [48] and dentine smear layers [49] appear similar when examined with scanning electron microscopy, it is anticipated that considerable ultrastructural difference should exist between the two types of smear layers and should reflect the composition of the underlying hard tissue substrates from which they are derived. Despite our current knowledge on the ultrastructure of dentine smear layers, that of the enamel smear layer has not been elucidated. Dentine smear layers consisted of globular particles approximately 0.05-0.1 mm in diameter [50, 51] that were separated by water-filled channels [52]. These globular particulates probably represent fractured apatite crystallite aggregates that are burnished together by the denatured collagen remnants. Due to their higher organic content, acid etching of dentine smear layers may not result in their complete dissolution, with the possibility of entrapment of remnant minerals within the gelatinous, denatured collagen matrices [53]. A recent transmission electron microscopy (TEM) examination of the enamel smear layer by the authors showed that the latter is composed of pieces of fractured apatite crystallites that are tightly bound (e.g. by saliva glycoproteins) together to form a surface crust over the cut-enamel surface (Fig. 1.4). These fractured apatite chips are considerably larger and probably reflect the larger size of the original enamel apatite crystallites from which they are derived.
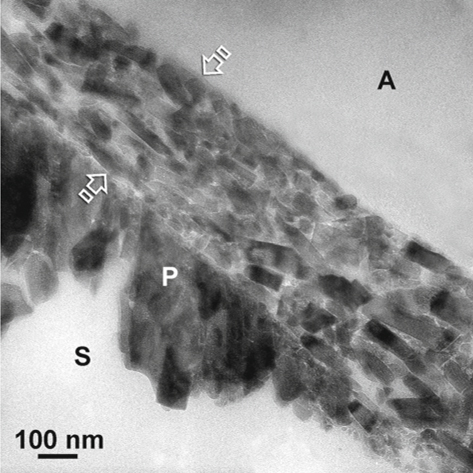
Fig. 1.4.
Under TEM, the enamel smear layer (between arrows) appears as fractured apatite crystallites that are burnished to form a crust over the cut prismatic enamel surface (P). A adhesive, S space
As smear layers are acid labile [54], the occurrence of an enamel smear layer did not present a challenge in bonding that involves the use of phosphoric acid etching; however, the presence of an enamel smear layer may become a potential problem when non-rinsing self-etch adhesives are used for bonding to bur-cut enamel. Similar to bonding with dentine smear layers, the less aggressive versions of self-etch adhesives must be acidic enough to etch through enamel smear layers to create micromechanical retention within the underlying prismatic enamel, with the creation of a hybridized complex [55] that consisted of a superficial hybridized enamel smear layer and a subsurface layer of hybridized prismatic enamel. For the more aggressive non-rinsing self-etch adhesives, it is likely that the enamel smear layer is completely dissolved or dispersed within the polymerized adhesive. As the inorganic content of enamel is higher than dentine, it is possible that enamel smear layers may have a higher buffering capacity for the acidic resin monomers than dentine smear layers [56]. This should be substantiated with further studies.
Application of Total-Etch Adhesives to Enamel
Unfilled bis-GMA-containing hydrophobic resins similar to those employed in pit and fissure sealants have traditionally been used for coupling of resin composites to etched enamel. Early studies in the 1970s showed that retention of resin composites to acid-etched enamel was independent of the use of an intermediate, unfilled coupling resin [57, 58–60]. Irrespective of their viscosities, resin tags that were formed by either resin composites or unfilled hydrophobic resins into the etched-enamel prisms were all in the range of 5-10µm. These studies were all performed in an era in which the concept of hybridization of dental hard tissues was unknown to the research community. Even at that time, Tang et al. [61] pointed out an important relation between the viscosity and adhesive penetration in etched enamel. They showed that adhesives with low viscosity produced both inter- and intraprismatic penetration in enamel after polymerization. Conversely, highly viscous adhesives result in the former type of penetration only. This difference in penetration of the adhesive in enamel was also reflected quantitatively in the tensile strengths of the resin-enamel bonds.
Several recent studies have shown that the use of a low-viscosity, unfilled resin is not necessary for effective coupling of orthodontic brackets to etched enamel [62–63]; however, only bond strengths to uncontaminated enamel were evaluated in those studies, without taking into consideration the effect of moisture contamination of the etched enamel during bracket bonding [64], or the ability of resin-sealed enamel to resist demineralization that could be induced by acidic beverages [65], or acids derived from plaque retained around brackets. It has been shown that in the absence of adjunctive fluoride treatment, significant protection against enamel demineralization may be achieved when acid-etched or hypomineralized enamel tissues were sealed with pit-and-fissure sealants, unfilled resins or dentine adhesives [66–70]. The results of these studies were also confirmed by Kuhar et al. [71]. Using electron paramagnetic resonance to monitor the diffusion of labeled molecules through acid-etched enamel, they showed that enamel permeability in both unetched bur-cut enamel and acid-etched enamel was substantially reduced with the application of a dentine adhesive such as Scotchbond Multi-Purpose Plus (3 M ESPE) to these exposed surfaces.
Bonding to Phosphoric Acid-Etched Cut Enamel
With the advent of the total-etch technique, it was natural to bond to etched enamel and dentine simultaneously with low-viscosity, solvented dentine adhesives that utilized hydrophilic monomers in primers. Jedrychowski et al. [72] demonstrated that a greater resistance to dislodging of resins by shear forces could be achieved when an NPG-GMA type of adhesion promoter was used on acid-etched human permanent enamel. Nakabayashi et al. [26] and Hotta et al. [73] found that in the absence of hydrophilic adhesion promoters, penetration of MMA-TBB resin into acid-etched enamel was in the range of 10 μm. The depth of resin penetration, thus, was similar to the results obtained previously with either unfilled hydrophobic resins or the use of resin composites alone. Conversely, when hydrophilic monomers, such as Phenyl-P or 4-META, were incorporated into the hydrophobic MMA-TBB resin, the depth of resin penetration increased to 16 and 23 μm, respectively. Improved resin penetration into etched enamel was also achieved when HNPM was added to TEGDMA in an orthodontic adhesive [74]. A recent study showed that enamel bond strengths achieved with the use of most total-etch single-bottle adhesives were at least equal to that of a conventional unfilled resin [75]. The use of dentine primers did not exhibit an adverse effect on long-term enamel bond strength and marginal adaptation when compared with enamel bonding resins; however, care must still be exercised when these primers are used on etched enamel that has been contaminated with saliva [76].
Most of these adhesives contain solvents that could displace residual moisture from acid-etched enamel and increasing resin penetration, allowing enamel to be bonded in the presence of moisture contamination. As the moist-bonding technique is required for bonding of most total-etch adhesives to dentine [77], it is difficult and impractical to bond to dentine and enamel simultaneously by keeping dentine moist and maintaining etched enamel dry at the same time. Recent studies have shown that while most total-etch adhesives were not affected by the presence of moisture on the etched-enamel surfaces [78–80], bonding to enamel contaminated with moisture has been poor in the absence of some of these adhesives [154]. There were also some adhesives that achieved higher bond strengths when bonding was performed on moist enamel [79, 81].
The incorporation of hydrophilic resin monomers in contemporary total-etch dentine adhesives allows optimal infiltration of the acid-etched enamel, promoting the hybridization of enamel and better inter- and intra-crystallite resin encapsulation. The hybrid layers created by these dentine adhesives on etched enamel are rendered more acid-resistant when compared with unbonded enamel. This can be readily appreciated when adhesive-infiltrated enamel was acid rinsed to bring these hybridized enamel tissues into relief prior to scanning electron microscopy (SEM) examination, or when undemineralized resin-enamel interfaces were demineralized in acids for observing the thickness of the enamel hybrid layers; however, such an acquired acid resistance should be regarded as relative, as demineralization of the resin-enamel interfaces with either EDTA or formic acid resulted in complete dissolution of the mineral phase that were trapped within the hybrid layer, and only stained enamel proteins could be identified. As these specimens were demineralized en bloc before embedding with epoxy resin, apatite crystallites that were encapsulated by the adhesive resin should be present if hybrid layers were truly impermeable. This may be partially explained by the presence of hydrophilic resin monomers in the adhesive that have an affinity for water via hydrogen bonding [82]. Although resin infiltrated, acid-etched enamel is generally thought to be more acid resistant and offers significantly better protection against subsequent demineralization when compared with unbonded enamel, the presence of these water channels within the resin-bonded enamel may account for the occasional lack of protection when adhesive-bonded cut enamel is subjected to artificial caries challenge (Fig. 1.5).
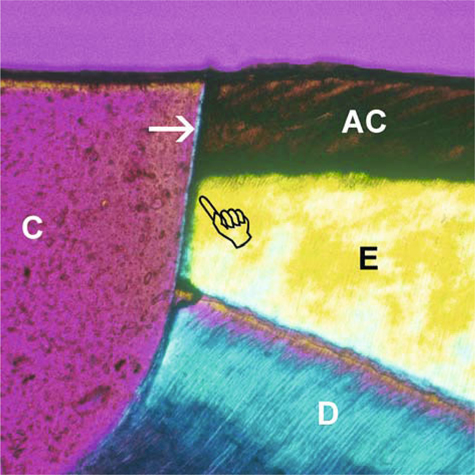
Fig. 1.5.
Polarized photograph of the resin-enamel interface in Single Bond (3 M ESPE) after artificial caries challenge in the absence of adjunctive fluoride protection. A thin-wall lesion (pointer) extends from the superficial artificial caries zone (AC) into the sound enamel (E) beneath the cavo-surface margin. Arrow :adhesive; C composite, D dentine
Bonding to Phosphoric Acid-Etched Uncut Enamel
The ability of total-etch adhesives to bond to less retentive aprismatic enamel enables aesthetic clinical procedures, such as porcelain veneers, to be performed [83]. Despite the lack of difference between bond strengths in ground and intact enamel after phosphoric acid etching [84, 85], the ultrastructure of the resin-enamel interface in phosphoric acid-etched uncut enamel remains the most variable and by far the most difficult to interpret, due to the mélange of aprismatic and prismatic etching features along the same interface [86]. Much of this depends on whether the phosphoric acid is applied with or without agitation (i.e. dynamic vs static etching). This is comparable to dynamic and static priming with respect to the application of self-etching primers on enamel [87]. Generally, the surface aprismatic enamel is more resistant to etching due to the parallel arrangement of the apatite crystallites which permit a high packing density of these crystallites. There is also no interprismatic organic substance that acid can readily diffuse through to effectuate subsurface etching. Static etching results in the retention of the bulk of the aprismatic enamel which demonstrates a less aggressive, coral-like etching pattern that is characterized by the presence of random-occurring surface pits on the surface of the etched aprismatic enamel. Depending on the thickness of the original layer of aprismatic enamel, only sporadic islands of etched aprismatic enamel may appear on the surface (Fig. 1.6), with the underlying prismatic enamel exposed to a greater extent. Nevertheless, the increase in microporosities created along the etching front as well as the subsurface creates a high-energy surface which is optimal for resin infiltration and results in hybrid layers that are 8-10 µm thick (Fig. 1.7).
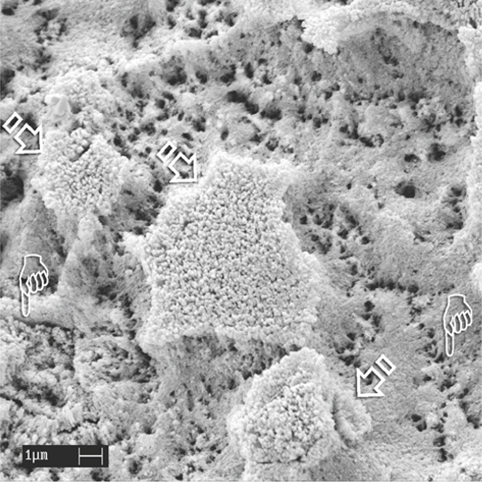
Fig. 1.6.
An SEM image of uncut enamel after static application of 32% phosphoric acid for 15 s. The bulk of the aprismatic enamel is dissolved, exposing the underlying etched, porous prismatic enamel with recognizable prism boundaries (pointers). Remnant islands of porous aprismatic enamel are still observed (arrows).
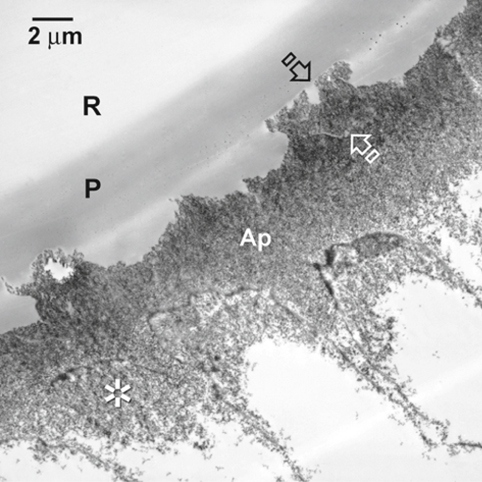
Fig. 1.7.
A TEM image of the resultant hybrid layer after the application of All-Bond 2 (Bisco). Remnant islands of aprismatic enamel (between arrows )are seen. The enamel hybrid layer consists of both aprismatic (Ap) and prismatic enamel ( asterisk). P primer, R resin
Dynamic etching brings fresh acid to the etching front, helps to dislodge loosened islands of etched enamel, and results in almost complete removal of the surface aprismatic enamel. This process exposes more of the underlying prismatic enamel that demonstrates minimal or mild etching patterns (Fig. 1.8). Even so, a high degree of variation exists between the two methods of acid application which is dependent upon the original thickness of the surface aprismatic enamel layer. It is generally understood that the formation of resin tags are minimized in phosphoric acid-etched uncut enamel, and that the predominant mode of micromechanical retention is achieved via the creation of surface and subsurface microporosities that result in an admixed zone of enamel hybridization (ca. 8-10 μm thick) consisting of both aprismatic and prismatic enamel (Fig. 1.9). Dissolution of the surface apatite crystallites results in preferential dissolution of the carbonate-rich crystallite cores (Fig. 1), forming central hole regions [88–89] that permit intra-crystallite resin infiltration [37].
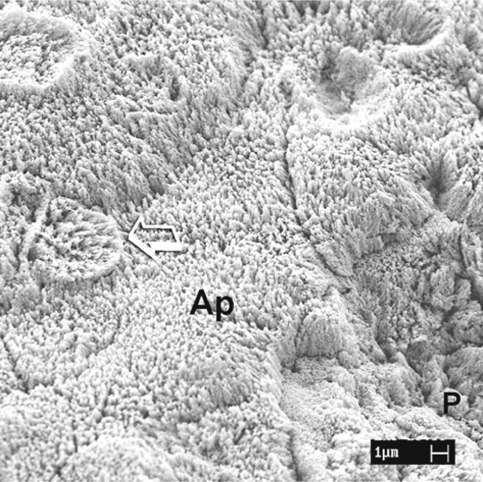
Fig. 1.8.
An SEM image of uncut enamel after dynamic application of phosphoric acid. The aprismatic enamel (Ap) is almost completely dissolved, with partial exposure of the rod heads of the prismatic enamel (arrow) . Complete dissolution of the aprismatic enamel on the right side reveals differentially etched enamel prisms (P)
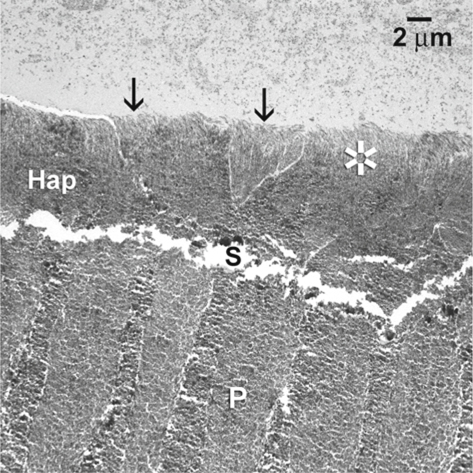
Fig. 1.9.
The TEM images of the corresponding hybrid layer (Hap), showing the concurrent presence of aprismatic ( asterisk) and prismatic enamel (arrows) along the surface of the hybrid layer. S space between the hybrid layer and the underlying unetched prismatic enamel (P)
Bonding to Primary Enamel
Due to the thicker layer of aprismatic enamel in the primary enamel [57], early recommendations for etching primary enamel called for doubling the etching time that was initially proposed for etching permanent enamel (i.e. 2 vs 1 min); however, Simonsen [7] reported that there was no difference in the retention rate of pit-and-fissure sealants when primary occlusal enamel surfaces were etched for 120 vs 60 s. Since that time, recommended etching times for permanent enamel have been reduced to 15 s. Similarly, a shorter etching time of 15 s was found to be satisfactory on intact primary enamel [25, 78].Hosoya and Goto [90] reported that there was no difference between the appearance of prism structures by etching unground primary enamel for either 60 or 30 s. With further reduction in etching time, there was a higher incidence of the absence of prism structure after phosphoric acid etching.
The TEM micrographs of the hybrid layers in phosphoric acid-etched uncut primary enamel showed a thick layer of hybridized aprismatic enamel that was devoid of resin tags (Fig. 1.10). Conversely, resin-enamel interfaces in primary teeth with the aprismatic layer completely removed by grinding revealed the presence of resin tags and the underlying hybrid layer in prismatic enamel that is not unlike that observed in permanent enamel. No difference in bond strength-to-primary enamel was observed when different total-etch and self-etch adhesives were applied to primary enamel followed by thermocycling [91]. Similar results were reported by Shimada et al. [92]. These authors showed that, although the etching effect appeared to be more aggressive for primary enamel, there was no difference between bond strengths of primary and permanent enamel when they were bonded using a total-etch or a self-etch adhesive, when bond strengths were assessed using the micro-shear bond strength test. Schmitt and Lee [93] also reported that there was no difference in microleakage when three-step or two-step total-etch adhesives were used in the primary or permanent dentition.
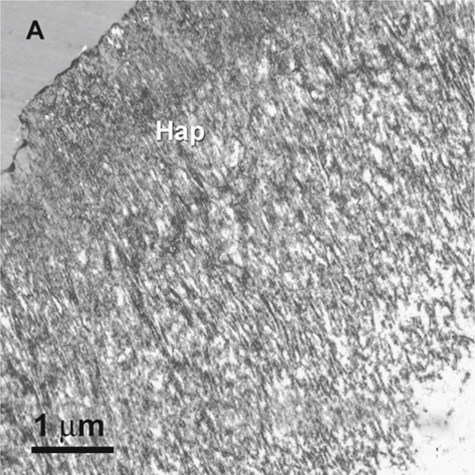
Fig. 1.10.
When All-Bond 2 is applied to phosphoric acid-etched uncut primary enamel, a hybrid layer is formed exclusively in aprismatic enamel (Hap). This is probably due to the thicker layer of aprismatic enamel in the buccal surfaces of the primary enamel. A adhesive (stained, demineralized TEM)
Application of Self-Etch Adhesives/Resin Cement to Enamel
Self-etch dentine adhesives and resin cements are becoming increasingly popular in restorative dentistry, preventive dentistry and orthodontics. With water being an integral component in these non-rinsing adhesives [35], the ambiguity in providing the optimal moisture condition for enamel and dentine bonding in the total-etch technique is eliminated [94]. Although enamel smear layers are devoid of smear plugs, post-operative sensitivity associated with the removal of the dentinal smear layer and smear plugs is also reduced when these non-rinsing adhesives are used for bonding to dentine [95]. The prospective uses of self-etch adhesives would be even more promising if they are equivalent in performance for clinical procedures such as the bonding of pit-and-fissure sealants [96] or orthodontic brackets [97–99],in which conventional phosphoric acid etching is still adopted as the mainstream technique; however, the use of self-etch adhesives is aggressively promoted by orthodontic manufacturers and is gradually gaining acceptance by clinicians, as it has been shown that a phosphoric acid concentration as low as 2% is adequate for bonding of brackets [100], with the additional benefit of preventing enamel damage when the brackets are removed at the end of treatment [101].
Aggressiveness of Self-Etch Adhesives
Unlike bonding to dentine, application of self-etch adhesives to enamel has been a controversial issue, particularly when mild self-etch adhesives are used on uncut enamel. Contemporary self-etch adhesive systems vary in their degree of aggressiveness which is dependent upon the concentration of the acidic resin monomers present, as well as the acidity (i.e. pKa) of the specific acidic monomers. This difference in aggressiveness influences the ability of self-etch adhesives to penetrate or dissolve enamel and dentine smear layers and to demineralize and bond to the subsurface bonding substrates [35]. The more aggressive versions can completely dissolve or disperse smear layers, forming thick hybrid layers in cut enamel and dentine that approach those achieved with conventional phosphoric acid etching. Conversely, the less aggressive versions incorporate smear layers as part of the bonded interface, forming only thin hybrid layers in intact dentine or enamel that are less than 1-2 µm thick. For bonding to uncut enamel, the efficacy of self-etch adhesives is dependent upon their ability to demineralize the more acid-resistant aprismatic enamel layer [36, 37]. Yoshiyama et al. [102] and Hara et al. [103] reported that bonding of self-etching adhesives to ground enamel was inferior when compared with single-bottle and multiple-step, total-etch systems which utilize phosphoric acid as a separate conditioner. Whereas some studies supported the manufacturers’ recommendations that the adjunctive use of phosphoric acid etching is necessary when bonding to this substrate [37, 84], others showed that there were no differences among the bond strengths of mild self-etch and total-etch adhesives to unground enamel [85, 92, 103–105].Although well-defined enamel etching patterns and resin tag formation are not prerequisites for achieving strong initial enamel bonds [37, 106], they have been associated with the stability [107] and improved survival rate of enamel bonds created in vivo. Although the adhesion-promoting ability of contemporary, mild self-etch adhesives may be equivalent to that of phosphoric acid-etched enamel, the thin lamina-like resin penetration produced on unground enamel with mild self-etch adhesives may not sustain cyclic stresses as favorably as deeper resin infiltration in both aprismatic and prismatic enamel that is promoted by the use of more aggressive self-etch adhesives or phosphoric acid etching [108]. A significant decline in enamel bond strengths was observed for some mild self-etching primer systems such as Imperva Fluoro Bond (Shofu), Clearfil Liner Bond II (Kuraray), and Mac Bond II (Tokuyama) after thermal cycling. Conversely, no significant decrease in enamel bond strengths was observed for some total-etch single-bottle adhesives to enamel after thermal cycling. Another important factor that influences the longevity of bonds made by mild self-etch adhesives on enamel is the ability of thin resin-enamel interfaces to resist fatigue stresses. A recent study by Nikaido et al. [109] on the bonding of Clearfil Liner Bond II to bur-cut dentine with thick smear layers reported a substantial decline in microtensile bond strength after fatigue load cycling. Similar tests should be performed in the future to evaluate the bonds made by mild self-etch adhesives to enamel.
Bonding to Cut Enamel
Studies on the bonding of self-etch adhesives to smear layer-covered dentine and enamel were traditionally performed by manufacturers on thin smear layers that were created using 600-grit silicon carbide papers; however, smear layers produced clinically are thicker but can be approximated by using 180-grit SiC paper [110]. Although contemporary self-etch adhesives have been improved considerably by increasing the concentration of acidic resin monomers in their composition [111–112], there is still the danger that they may be buffered by the mineral content of thick smear layers [56]. Recent reports have suggested that some of the less aggressive versions of self-etching primers failed to etch through clinically relevant, thick smear layers produced by diamond burs [113], resulting in decreases in tensile bond strengths after fatigue load cycling [112]. Conversely, there was no drop in dentine bond strengths in specimens that were prepared using 600-grit silicon carbide papers; however, no data are yet available on the ability of self-etch adhesives to penetrate clinically relevant, thick enamel smear layers.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


