Introduction
Compression on the midface with nasal mask-delivered positive airway pressure (PAP) therapy in growing patients might contribute to midface retrusion. The objective of this study was to investigate the association between long-term PAP use and craniofacial morphologic pattern in children with persistent obstructive sleep apnea.
Methods
Images generated with cone-beam volumetric imaging were used to complete lateral cephalometric analyses of anteroposterior projection of the midface region. The study group included 12 subjects (10 boys, 2 girls; mean age, 9.0 years) who used PAP therapy for at least 6 months and at least 6 hours per night. Measurements from this group were compared with those of a control group of 11 subjects (5 boys, 6 girls; mean age, 9.6 years) with obstructive sleep apnea who did not have PAP. Measurements were taken at 1 time point.
Results
No significant differences were identified between the groups for any cephalometric variable. Multivariate linear regression analysis also did not identify a significant association between the number of months of PAP therapy and the cephalometric variables. Cephalometric data for both groups were pooled for comparison with appropriate published normative values for age and sex. Anterior cranial base length, overall anteroposterior length of the maxillary base, and mandibular body length were significantly shorter than normal in the subjects compared with published normative values.
Conclusions
No association was demonstrated between midface projection and PAP use in growing patients. When compared with normative data for anterior cranial base, children with obstructive sleep apnea had shorter maxillary and mandibular lengths.
Obstructive sleep apnea (OSA) is a form of sleep-disordered breathing characterized by recurrent episodes of partial or complete airway obstruction during sleep. The reported prevalence of OSA in children varies by age range, method of assessment, and definition of OSA. Recent studies using polysomnography in elementary school children have reported prevalence rates of 1.0% to 2.2%. The pathophysiology of OSA in any patient depends on the specific primary etiologic factors, but the overarching predominant mechanism in noncentrally mediated OSA is physical occlusion of the upper airway due to an anatomic obstruction. This obstruction can occur anywhere from the nares to the epiglottis, but in children the obstruction most commonly appears to be a result of adenotonsillar hypertrophy. Other risk factors include enlarged nasal inferior turbinates, nasal septum deviation, chronic sinusitis or rhinitis, allergic rhinitis, unfavorable craniofacial skeletal morphology and growth patterns (midface dysplasia or mandibular retrognathia), obesity, and some neuromuscular disorders.
Children diagnosed with OSA have limited treatment options. The first-line surgical treatment for pediatric OSA is adenotonsillectomy, with a reasonable success rate and significant improvement in quality of life postoperatively. Pharmacologic management options include the use of topical or systemic corticosteroids in addition to anti-inflammatory agents for chronic nasal mucosal inflammation. In patients whose midface hypoplasia is thought to be a major contributor to the etiology of upper airway resistance, orthodontic protraction with or without maxillary expansion using surgical or nonsurgical means might be curative. Orthognathic surgical options include maxillary expansion, maxillary or mandibular advancement, and osseodistraction. Other surgical options include uvulopalatopharyngoplasty, tongue base reduction, lingual tonsillectomy, and in severe refractory cases, tracheostomy.
Postitive airway pressure (PAP) therapy with a nasal mask can be used as a bridge to surgical therapy or as the primary treatment for patients who have no clinical evidence of adenotonsillar hypertrophy or are unresponsive to adenotonsillectomy, or when surgical procedures are contraindicated.
For nasal mask-delivered PAP devices to be effective, an airtight seal between the nasal mask and the perinasal area must be achieved. A tight seal is obtained by ensuring adequate pressure and fit by the mask flanges against the facial skin with the headgear straps. The pressure exerted by the mask on the soft tissues and the underlying growing and remodeling bones of the midface might be a cause of midface retrusion in growing children on long-term PAP therapy during their peak growth years. If this association exists, it would be safe to assume that its magnitude and impact would be directly related to the length of the PAP therapy, the patient’s compliance, the magnitude and direction of pressure exerted on various areas of the face, and the timing of the initiation of treatment in relation to the degree of skeletal developmental maturation of the nasal-maxillary complex.
The potential iatrogenic orthopedic effects of long-term PAP use with a nasal mask have not been adequately investigated. A better understanding of the potential interactions between long-term PAP therapy and craniofacial growth and development is required before PAP therapy with a nasal mask can be considered for growing children as a viable and safe treatment from an orthopedic perspective. The objective of this study was to investigate the association between PAP use and craniofacial pattern in children with persistent sleep disordered breathing or OSA despite initial surgical treatment for the OSA (adenoidectomy).
Material and methods
Participants in this study were recruited from the patient pool of the Pediatric Sleep Medicine Program at the University of Alberta Hospital in Edmonton, Alberta, Canada. Ethics approval was obtained from the University of Alberta’s Health Research Ethics Board. Informed consent was obtained from all participants and their guardians.
With the exception of PAP status, the same selection criteria were used for both the study and control groups, including (1) age, 6 to 18 years; (2) white ethnicity; (3) OSA diagnosed by a sleep medicine specialist (based on standardized polysomnographic, clinical, and other diagnostic criteria); (4) adenoidectomy (with or without tonsillectomy) completed; (5) no previous orthodontic or orthognathic surgical treatment; and (6) no craniofacial syndrome.
Consecutive patients were assessed for suitability for the study as they came to the Pediatric Sleep Medicine Program over a 9-month period based on referral from other practitioners or follow-up from prior evaluations in the sleep clinic. Those who met the inclusion criteria were asked to participate in the study. Subjects who had not received PAP therapy became the control group. Those who were treated with nasal PAP therapy for 6 months or more were allocated to the study group. The sample size for comparing the study and control groups was determined to be 10 per group (total of 20 subjects) with the large effect size, significance set at 5%, and power at 70%.
All participants received a 3-dimensional radiographic examination with cone-beam volumetric imaging with an i-CAT machine (Imaging Sciences International, Hatfield, Pa) with the full field of view setting of 13 cm. The images were obtained in the DICOM3 format and processed using Dolphin 3D software (Dolphin Imaging & Management Solutions, Chatsworth, Calif) to produce 2-dimensional lateral cephalometric and panoramic images according to a standardized imaging protocol.
The cephalometric analysis used in this study was based on a combination of the most commonly used variables and specifically selected measurements describing the anteroposterior projection of the midface region in the sagittal plane. A list and a description of the variables in our cephalometric analysis used in this study are shown in the Figure and Table I .
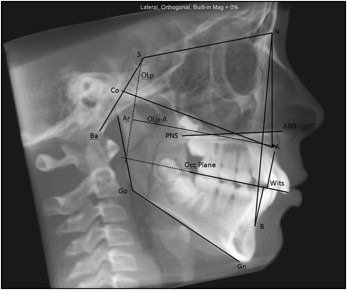
| Cranial base | |
| S-N (mm) | Length of anterior cranial base |
| BaSN (°) | Angle of flexure of cranial base |
| Maxilla/midface | |
| SNA (°) | Sella-nasion–A-point: maxillary anteroposterior projection |
| PP-SN (°) | Palatal plane-SN: vertical inclination of palate relative to cranial base |
| Co-ANS (mm) | Condylion-ANS: maxillary anteroposterior projection |
| ANS-PNS (mm) | Anterior nasal spine-posterior nasal spine: length of palate |
| U1-PP (°) | Angulation of maxillary incisor to palatal plane |
| A-NPerp (mm) | A-point-perpendicular to Frankfort horizontal at N: maxillary projection |
| OLp-A (mm) | Linear distance between A-point and a line drawn perpendicular to the occlusal plane at sella (OLp) |
| Mandible | |
| SNB (°) | Sella-nasion-basion: mandibular anteroposterior projection |
| ArGoMe (°) | Articulare-gonion-menton: angle of mandible |
| Go-Me (mm) | Gonion-menton: length of mandibular body |
| Maxilla-mandible | |
| ANB (°) | A-point–nasion–B-point: relative position of mandible to maxilla |
| Wits (mm) | Distance between perpendiculars to occlusal plane at Points A and B |
All lateral cephalometric images were traced by the same operator (M.M.K.), who is an experienced orthodontist. To reduce the effect of operator measurement error, the tracings were made at 3 separate times per patient in random order, and the values for each tracing were entered into an electronic spreadsheet (Excel, Microsoft Office 2010; Microsoft, Redmond, Wash). Mean values for each cephalometric variable from the 3 tracings per image were computed and used for the analysis. Blinding of the evaluator to the identities of patients and study groups to which the images belonged was maintained throughout this process with a coding system.
Statistical analysis
Intraoperator reliability was evaluated for all cephalometric variables using the intraclass correlation coefficient. The nonparametric Mann-Whitney U-test was used for comparisons between the subject and control groups. Additionally, multivariate linear regression analysis and the Pearson correlation analysis were used to assess potential associations between length of time in PAP therapy (months), compliance with PAP therapy, and variations in cephalometric variables. All statistical analyses were performed using SPSS software (version 16.0; IBM SPSS Statistics, Armonk, New York).
The cephalometric variables for both groups were evaluated against published normative data. The raw data for the normal group were not available, but the mean and the standard deviation for each variable were. The t test statistic for comparing 2 groups (PAP vs norm; control vs norm) was calculated by hand with this formula:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


