Computers are used increasingly as a supportive tool for diagnosis, operative planning, and treatment in medicine and dentistry. They are used in combination with ultrasound and digital imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) to improve the visualization of anatomical and physiological conditions. Almost every medical specialty shows a tendency toward less invasive procedures, and good imaging is essential for diagnosis and surgery, particularly for minimally invasive procedures. Within our own surgical specialty, this extends into all areas, from dental implantology to the treatment of craniofacial malformations and advanced tumors. It is particularly important in this complex anatomical maxillofacial region.
Whereas in diagnostic imaging enormous progress has been made, the intraoperative use of imaging techniques has been relatively restricted. Intraoperative three-dimensional (3-D) imaging with ultrasound, CT, and MRI is still costly and is available only at an experimental level and for exceptionally complex cases. Computer-assisted surgery will be a help in this field in the future, and techniques of virtual reality will become increasingly important in relation to medical devices.
The goal of the interactive, intraoperative application of 3-D imaging has been used in part through instrument navigation systems. These systems enable the surgeon, for the first time, to show the actual instrument position in the surgical site on the 3-D reconstructed image dataset of the patient. It is also possible to focus on the position of a pathological or anatomical structure within the operative field. “Mirroring” of the non effected side is invaluable in trauma reconstruction.
Diagnostic Imaging
3-D imaging techniques such as CT, conebeam computed tomography (CBCT), MRI, and ultrasound can present almost every anatomical and pathological structure with high resolution and quality. Present developments concentrate on artifact reduction and on techniques for automatic fusion of the various imaging modalities.
Segmentation Procedures
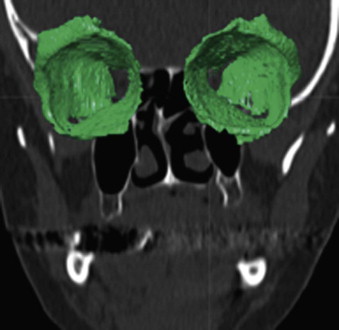
The segmentation procedure is necessary to mark anatomical structures for further data manipulation and planning. Hounsfield-specific units (e.g., delineating bone, soft tissue, muscles, tumors) on a 3-D dataset can be selected in each slice of the dataset or by defining the area of interest. Performing the segmentation slice by slice is very time-consuming, whereas defining the area of interest and performing a threshold segmentation by selecting specific Hounsfield units often is not sufficiently precise ( Fig. 32-1 ). In craniomaxillofacial surgery, the midface area with its thin anatomical bone structures often leads to the presence of virtual “pseudoforamina” or artifact holes after the segmentation procedure.
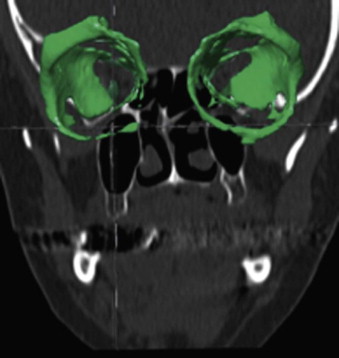
Because of these complications, modern software offers so-called automatic atlas segmentation procedures. After the data is imported into the planning software, a menu is available from which the surgeon can select the area of interest. A deformation algorithm transfers the information of a standarized and segmented skull atlas into the patient-specific dataset. After this procedure, the adapted parts can be used for further planning in the patient dataset ( Figs. 32-2 and 32-3 ).
Segmentation Procedures
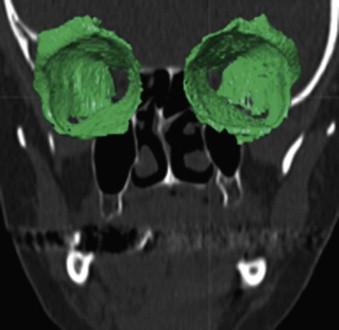
The segmentation procedure is necessary to mark anatomical structures for further data manipulation and planning. Hounsfield-specific units (e.g., delineating bone, soft tissue, muscles, tumors) on a 3-D dataset can be selected in each slice of the dataset or by defining the area of interest. Performing the segmentation slice by slice is very time-consuming, whereas defining the area of interest and performing a threshold segmentation by selecting specific Hounsfield units often is not sufficiently precise ( Fig. 32-1 ). In craniomaxillofacial surgery, the midface area with its thin anatomical bone structures often leads to the presence of virtual “pseudoforamina” or artifact holes after the segmentation procedure.
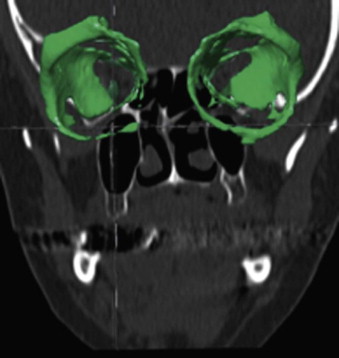
Because of these complications, modern software offers so-called automatic atlas segmentation procedures. After the data is imported into the planning software, a menu is available from which the surgeon can select the area of interest. A deformation algorithm transfers the information of a standarized and segmented skull atlas into the patient-specific dataset. After this procedure, the adapted parts can be used for further planning in the patient dataset ( Figs. 32-2 and 32-3 ).
Planning and Simulation
Automated generation of the proposed operation with simulation of surgical procedures and outcome is nearly possible within the framework of virtual surgery. For the simulation of operative results (i.e., virtual 3-D graphic simulation of various treatment alternatives on the computer), software must be developed to allow for interactive manipulation. This should be in real time within the 3-D visualization, including viewing from various angles, cutting, palpation, and insertion of implants. Here, the user’s interaction has to be conform with the usual surgical actions. In the future, input media with power feedback (haptic interfaces from the field of virtual reality technologies) will offer options for intuitive control of complex 3-D simulation environments.
Intraoperative Support
The imaging data acquired preoperatively during diagnosis should be available for interactive use by the surgeons at all times. In this way, modern techniques of computer-assisted surgery can help to decrease the operative risks and postoperative morbidity rates. Future interactive support for the surgeon can be characterized within three areas:
- •
Passive tools for support of the intraoperative orientation will allow for routine transfer of the preoperative plan onto the patient. These tools include projection techniques, head-mounted displays, and instrument navigation systems.
- •
Guiding systems (semiactive manipulator systems) will show the surgeon a risk-free path for the surgical instruments to achieve the operative plan. By this means, operative strategies can be transferred to the surgical site precisely and safely.
- •
Surgical robots will execute specific operative steps In this case, the surgeon will leave control to the robot for certain parts of the operation. The best-known example is the highly precise milling of the femur shaft for adaptation of a hip joint endoprosthesis, which is already in routine clinical use.
Intraoperative Imaging
Intraoperative imaging is most helpful for maxillofacial procedures that require precise postoperative symmetry with limited intraoperative visualization. If the surgeon would consider obtaining a postoperative CT to check implant placement or symmetry, then intraoperative imaging could also be considered. It will provide the surgeon with the required anatomical information in the operating room and allow for a revision if necessary.
There are two modalities for intraoperative CT scanning: fan beam scanners (CT) and conebeam scanners (CBCT). Both are rapid (requiring <2 minutes per scan) and give adequate bone resolution. Due to better handling, lower costs, and less x-ray exposure, intraoperative imaging by CBCT (C-arm machine) has won recognition.
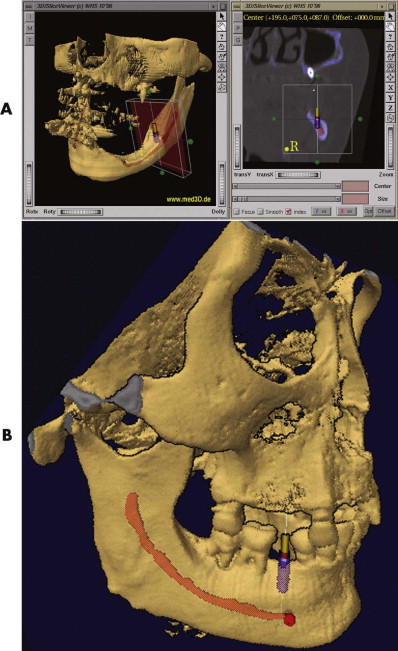
The C-arm provides 3-D reconstructions that are acquired by rotation around an isocentric point. With panning of the C-arm over the surgical field, no reposition of the patient is necessary. The acquired 3-D data can easily be imported into the existing planning software of the navigation device. With the use of image fusion, the preoperative plan can be compared with the intraoperative imaging data ( Fig. 32-4 ). This shifts the postoperative control into the operating room, decreasing complications, and costs.
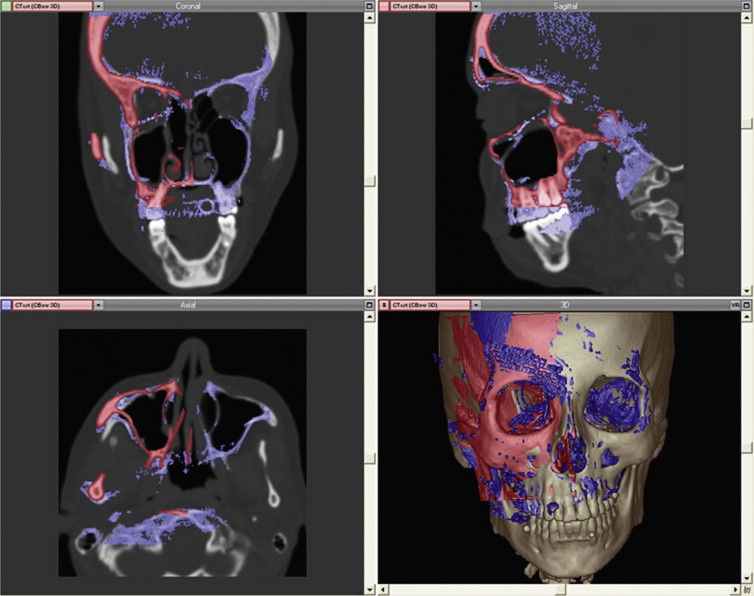
Postoperative Evaluation
Postoperatively, a comparison between the control imaging data and the original imaging data for evaluation of the real surgical result will permit scientific studies using the follow-up controls. The resulting data can be used for optimization of operative strategies using the software tools to improve prediction of the surgical outcome.
Diagnostic Imaging Applications
The existence of suitable imaging data in the form of 2-D and 3-D datasets is critical for successful preoperative planning and intraoperative navigation. In many cases, we use several, partly complementary imaging devices in the same anatomical area to obtain detailed, supplementary clinical information.
CT offers the advantages of a precise, reproducible and high-contrast presentation of osseous structures. The image quality of CT is diminished by patient movement, as well as the presence of metal artifacts, and if there is limited soft tissue contrast. Advantages of MRI are its excellent soft tissue imaging choice of image planes, and the possibility of functional imaging (diffusion- and perfusion-weighted acquisitions, magnetic resonance angiography). In addition, the patient is not subjected to radiation exposure. Disadvantages are the various artifacts (e.g., distortion), both appliance specific and patient induced, including the chemical shift, susceptibility artifacts, movement, and metal artifacts.
Sonography (ultrasound) offers the advantages of quick access, high resolution, the choice of image planes, and especially the possibility of tracking anatomical structures and determining function (e.g., blood flow, and vascularity). Disadvantages are the dependence on the examiner’s skill and the geometrical distortions and associated “noise”. The use of 3-D sonography creates more indications, with the possibility of reconstruction of the image layers parallel to the CT or MRI images. In addition it may be aligned to give the view for the surgeon, resulting in improved orientation.
The possibility of intraoperative use offers an additional advantage of sonography and, to a lesser extent, of CT and MRI. Imaging must be considered not only with respect to diagnosis but also in connection with operative planning and other methods based on these data. Especially important are the imaging parameters chosen by the surgeon in cooperation with the radiologist, such as the plane of the cuts, layer spacing and thickness, and use of contrast media.
Because of the increasing need to include all imaging information in treatment planning, it is desirable sometimes to combine the data from various sources to achieve a fused imaging dataset ( Fig. 32-5 ). For this purpose, an automated fusion of the various image modalities and automated segmentation of the anatomical and pathological structures should be available but is not yet possible with the present state of the art. So far, only bones and skin surfaces can be segmented automatically using CT data. However, knowledge of the exact positions of “at-risk” structures such as vessels and nerves is especially important—for example, to avoid damage during the planning phase of the operative steps to be performed by a robot.
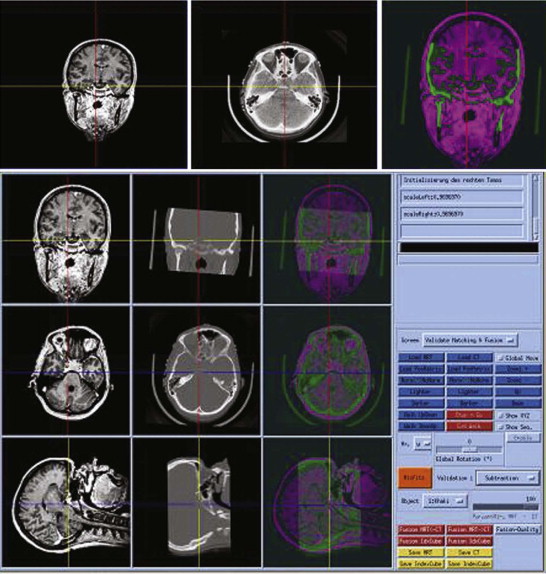
Many implantologists wish to have a 3-D visualization of the bone status for planning of complex cases. So far, CT has been used infrequently as a diagnostic aid, not least because of the associated radiation exposure. However, dental implantologists are interested primarily in bony structures, and high soft tissue resolution is not necessary. Dose reduction seems promising. Dose-reduced CT allows exact metric measurements in the range of 1 mm or less as well as 3-D representation of the jawbone, facilitating a precise 3-D implantation plan with justifiable radiation exposure. Therefore, we consider CT-assisted planning to be indicated before dental implantations in patients with limited bone volume, for example near the maxillary antrum or the mandibular canal, and when multiple implants are planned.
Following technical developments, 3-D radiographic techniques are expected to become increasingly important in oral surgery. CBCT scanners produce a 3-D imaging data record without the need to move the patient table. With the conebeam technique, dedicated devices can be built especially for dentistry; in design, these could be similar to conventional devices ( Fig. 32-6 ). It seems realistic to expect the development of further devices with costs comparable to those of current orthopantomography devices.
Planning and Simulation Applications
In contrast to the rapid progress in diagnostic imaging, development and clinical use of new technologies for planning and performing therapy are lagging behind. This can clearly be seen from the fact that 3-D CT imaging data are still exposed as 2-D images on X-ray film. Analysis is mostly restricted to general statements or a few measurements. Planning is carried out mentally by the treating physicians and depends very much on their experience and powers of imagination.
The aim of operative planning in oral and maxillofacial surgery is optimization of the surgical result in terms of function and esthetics. The prerequisite for successful operative planning is preparation of preoperative patient imaging data. For segmentation of the original imaging data, individual pixels or voxels are assigned to certain classifications such as skin, bones, at-risk structures, and tumors. Powerful graphic workstations are necessary for interactive manipulation in great detail.
With the aid of the patient model generated from the original imaging data, the operative goal can be defined and the operation planned. However, the model is only an approximation of the patient. Certain information, such as that contained between the layers or “slices” of the imaging data, is missing, so precision is limited by layer thickness.
At the present time, there is a serious discrepancy between the scientific development of tools with highly complex software systems and the simple handling requirements the surgeon uses. This problem prevents the conversion of a theoretical process into practical application, because the interactive planning of a complex bone-shifting operation requires more than 1 hour.
Optimal use of new technologies to safely plan and carry out surgical interventions requires an operative planning system that can be consulted by the surgeon during preoperative planning, intraoperative intervention, and postoperative evaluation. During preoperative planning, the first task is to define the operative aim. For example, the bone segments to be moved must be specified and virtually cut, moved, and positioned.
Two application areas are described here to highlight the clinical importance of preoperative planning and simulation.
Computer-Assisted Planning in Dental Implantology
Conventional preimplantation planning is usually conducted with a 2-D panoramic radiograph. This method cannot supply an assessment of the actual bone volume in a buccolingual dimension. Often, it becomes apparent during surgery that the bone is too narrow or is not suitable for an implant due to concave cortical surfaces. When the third dimension is unknown, large distances must be left between implants and neighboring structures for safety reasons, and the use of all the available bone cannot be optimized. CT-based planning systems for dental implantology have been introduced with the goal of overcoming these limitations. To choose the most suitable implant insertion site, it is possible to move through an almost unlimited succession of finely graduated cross-sectional and panoramic sections. At any time, the treating physician can reconstruct the exact information required for treatment planning without having to reexamine the patient. These software-generated secondary sections from the CT dataset of a jaw allow, for the first time, the mucosal surfaces and evaluation of the vestibulo-oral dimension of the alveolar process and immediate metric registration directly on the screen. However, most software systems commercially available today have one serious disadvantage: real time 3-D visualization is not possible.
Software programs for interactive 3-D planning of dental implantations have only recently become available. This data provides high resolution, and corresponding detailed visualization of the patient’s bone volume data. Therapy planning additionally requires the representation of virtual surgical equipment such as implants, drills, and saws which can more precisely utilized. For operator convenience, frame rates of several images per second are necessary; only then will intuitive and interactive positioning of the implants possible.
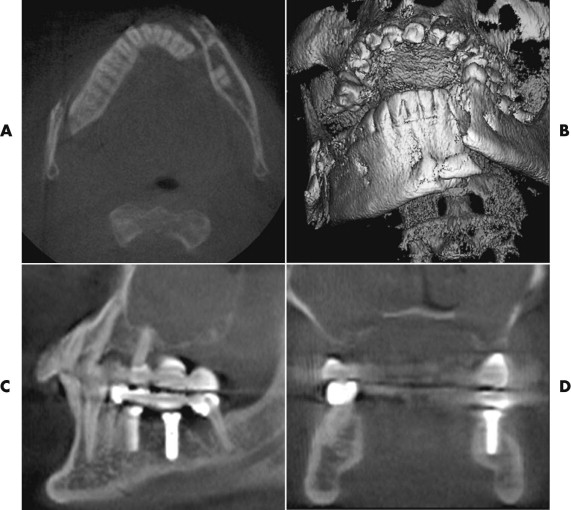
A system for interactive 3-D planning for dental implantology, developed at our hospital ( Fig. 32-7 ), allows the 3-D positioning of implants with high image quality. The position of the mandibular canal requires only identification of the mandibular foramen and the mental foramen; from that, the software automatically performs an analysis of the most probable course of the mandibular canal within the CT image. Based on bone availability, the distance to critical structures, and the planned prosthetic superstructures, the surgeon decides on the position of the implants.
From a prosthetic view, to prevent interferences between the optimal number, position, angulation, and type of implants and the existing anatomy, it is necessary to match the preoperatively obtained data (bone quality and bone quantity) with prosthetic demands (statics, dynamics, and esthetics) during the planning. The successful result of an implant superstructure can be optimized and guaranteed for the long term. Future developments in this area will also encompass the integration of 3-D simulation of prosthetic superstructures and their esthetic effects into the planning software environment. In this way, the virtual implantation procedure, from a prosthetic and surgical viewpoint, will be achieved for the first time.
The advantages of CT-based implantation planning do have to be considered in relation to the disadvantages of availability of scanners, higher costs, and higher radiation exposure for the patient. Many authors consider it better to apply standard orthopantomography techniques for routine examinations and to reserve CT-based implantation planning for particularly complex cases, such as those with reduced bone volume, especially near the maxillary sinus and mandibular canal, and for planning the insertion of multiple implants.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


