Fig. 4.1
Traditional tray showing (a) the evident possibility of direct contact of the bleaching gel (arrow) with soft tissue and (b) the scalloped tray, 0.5 mm short of the gingival tissue line, that allows keeping the bleaching gel in contact exclusively with enamel
In a clinical study conducted by Leonard et al. (2001), a 10 % CP gel applied daily (6–8 h) for 14 days using scalloped trays resulted in minimal adverse effects on oral soft tissues. The authors conducted an analysis of the marginal (gingival index) and nonmarginal gingiva (nonmarginal gingival index), and the possible changes in non-gingival soft tissues (non-gingival oral mucosal index) after 7 and 14 days of treatment. They did not observe any significant differences in all the parameters tested between the bleached and nonbleached groups. Only 8 % of the patients reported gingival irritation during the treatment course, and none of them reported gingival irritation during 3, 6, and 47 months posttreatment. Similar results were found in the study by Almeida et al. (2015) where the application (2 h daily) of bleaching gels containing 10 and 16 % CP in scalloped trays for 21 days did not cause genotoxicity in the adjacent gingival tissue to the teeth subjected to bleaching. In pre- and postbleaching analyses, Firat et al. (2011) observed that the application of 35 % CP gel at home in trays with gingival trimming for 15 days (30 min/day) caused no changes in clinical parameters for the gingival tissue and periodontium, but increased levels of proinflammatory cytokines in crevicular fluid. A number of clinical studies reported that contact of at-home bleaching gels with oral tissues resulted in inflammation and erosion of the marginal gingiva, as well as cervical resorption and gingival recession, with the contact of the products with the marginal gingiva for long periods being contraindicated (Powell and Bales 1991; Haywood et al. 1997). Costa-Filho et al. (2002) performed a gingival tissue biopsy for smokers and nonsmokers who underwent treatment with a gel containing 10 % CP, applied for 8 h/day for a period of 5 weeks. Biopsies performed immediately after the bleaching treatment revealed an increase in epithelial thickness and cell proliferation to basal and parabasal layers, resulting in morphometric changes of the gingival tissue, when compared with biopsies performed 15 days before the bleaching treatment. The results were similar between the smokers and nonsmokers. In the abovementioned clinical studies, bleaching trays without gingival trimming and reservoir in the vestibular region were used.
More recent studies reported that the immediate efficacy of bleaching does not appear to be affected by the smoking habit (de Geus et al. 2015a, b, c). Additionally, tray whitening did not induce DNA damage to soft tissues during the treatment (de Geus et al. 2015c) or even increase the risk of tooth sensitivity (de Geus et al. 2015a).
The possibility of daily contact of bleaching agents rich in HP with oral tissues remains controversial. Several studies showed that HP in high concentrations might promote cancer in the duodenum and jejunum of rats treated concomitantly with carcinogens. However, HP administration alone did not lead to the development of lesions in these tissues (Naik et al. 2006; Minoux and Serfaty 2008; Paula et al. 2015). However, according to the results reported by Hannig et al. (2003), only 1.25 % HP present in the gel with 10 % CP was detected in saliva from patients who underwent bleaching in conventional individualized trays with a 1.5-mm reservoir, which was the highest release observed within the first 5 min. Thus, the systemic effects of HP derived from at-home bleaching are still considered quite controversial (Naik et al. 2006; Minoux and Serfaty 2008). It is noteworthy that a cocarcinogen agent does not start mutations alone but requires a neoplastic initiator in the oral cavity (Naik et al. 2006). The possibility of the correlation between a cocarcinogen agent and an initiator is a contraindication for carrying out aesthetic treatment.
Treatment supervision by a qualified dental professional is extremely important to diagnose early changes in hard and soft tissues. The indiscriminate online access to home bleaching materials and the use of OTC products from online retailers and conventional drugstores may increase the safety risks of tooth whitening.
While there are an abundant number of studies on CP-based gels for at-home application, few studies are available related to the effects of HP-based home gels on soft tissues. Several studies showed that the amount of HP in saliva is proportional to the HP concentration in bleaching gels and that the use of CP-based gels results in lesser amount of HP in saliva than products containing pure HP (Hannig et al. 2003, 2005). The decomposition of 10 % CP into HP has been demonstrated to occur primarily in the first hour after bleaching, and this degradation occurs primarily in the region of product contact with the tooth surface (Matis et al. 1999). However, current clinical evidence shows that a better bleaching outcome is obtained when 10 % CP is applied for 8–10 h (Matis et al. 2009; Cardoso et al. 2010), as seen in Chap. 6.
Thus, the application of a sufficient amount of bleaching gel (enough to cover the tooth surface) in a custom-fitted scalloped tray without reservoirs promotes effective tooth bleaching with minimal damage to oral soft and/or pulp tissue. Furthermore, in order to prevent inadvertent swallowing of bleaching product residues, home bleaching with the use of gels with 10 % CP applied using a scalloped tray model slightly short of the free gingival margin (≈0.5 mm) has been recommended.
The direct contact of bleaching gels with gingival and periodontal tissues should be avoided in order to eliminate the possibility of tissue damage mediated by HP.
4.2.2 OTC Products
Currently, another type of tooth bleaching treatment, which involves the use of OTC products, has become popular (see Chap. 6). OTC products can be purchased in supermarkets, drugstores, or even on the Internet, and are used without dentist supervision. These products emerged in the United States about 15 years ago as an alternative treatment of stained teeth with lower cost than traditional supervised treatment (Demarco et al. 2009). The active component is the same as that in traditional bleaching agents, that is, either CP (10–18 %) or HP (1.5–14 %), which is available in various forms such as bleaching strips, varnishes, gels, paint-on liquids, mouthwashes, and toothpastes. However, these products offer no protection to soft tissue adjacent to the teeth subjected to bleaching. As such, their indiscriminate use without professional guidance raises concerns about the possible adverse effects (Demarco et al. 2009).
Also available online are bleaching gels to use with universal trays, even those administered with lights and electrodes. Poor adaptation of the tray results in the flow of the material to the oral cavity, causing contact of a large amount of product with the oral mucosa and possible swallowing of high concentrations of toxic components. An OTC product was applied in areas of gingival recession (Ghalili et al. 2014), a procedure contraindicated especially when using prefabricated trays. Studies that used varnishes and paint-on liquids showed that these products do not promote effective bleaching of the tooth surface (Kishta-Derani et al. 2007; Lo et al. 2007). Furthermore, clinical evidence suggests that both at-home bleaching and in-office bleaching are equally efficient for bleaching teeth and are found to be superior to Whitestrips (Bizhang et al. 2009). We therefore consider that these materials, apart from having poor aesthetic effectiveness, may also cause some risk to the health of consumers (Fig. 4.2).
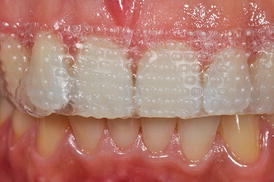

Fig. 4.2
Gingival tissue in contact with bleaching strips
Among the OTC bleaching products, bleaching strips are the most popular products owing to their aesthetic result, making them superior to other products of the same category available in the market (Xu et al. 2007; Yudhira et al. 2007; Kwon et al. 2013). These products were created to use without trays, with a thin layer of HP added to the adhesive surface that is released in relatively short periods (5–60 min). A systematic literature review demonstrated that there are differences in aesthetic effectiveness between the products due to the levels of active ingredients and that the whitening strips and products with high concentrations of HP caused more adverse effects (Hasson et al. 2006).
Bleaching gels with 10 % CP for at-home use contain 3.3–3.5 % HP in its composition, about half of the HP concentration found in the less-concentrated strips. Clinical studies have demonstrated that the HP concentration in the saliva of patients who underwent bleaching with strips (5.3 % HP) is about two to four times higher than the concentration in saliva observed for 10 % and 15 % CP gels applied in custom trays (Hannig et al. 2003, 2005).
As the bleaching strips have a predefined shape, contact of the HP-rich surface with the gingival papilla occurs during treatment. As discussed previously, adverse effects on gingival and periodontal tissues are expected, and the extent thereof may lead to the development of problems in patients, especially because the products are applied without dentist supervision, which may lead to its indiscriminate use.
The main concern regarding the safety of these bleaching strips is related to the absence of protection of the gingival tissues.
In an interesting study by Auschill et al. (2012), the bleaching efficacy and biological effects on soft tissues provided by bleaching agents with similar HP concentrations were evaluated. However, the products were applied according to the supervised at-home technique or by using a bleaching strip. Patients were instructed to use a home bleaching gel containing 5 % HP in a scalloped tray with a 1.0-mm reservoir or to apply a bleaching strip containing 5.3 % HP, without any method of protection of soft tissues, as recommended by the manufacturers. Both products were applied twice daily for 30 min during the 14-day period. The bleaching efficacy during, at the end, and 18 months after the treatment was statistically similar for both products. However, 40 % of the patients who used the bleaching strip reported soft tissue irritation, while only 20 % of patients who underwent bleaching with a tray reported the development of this adverse effect. Tooth sensitivity was also more prevalent in the patients who used a bleaching strip (60 %) than in those who used a gel in the tray (47 %). In both groups of patients, the adverse effects were considered mild and transient. Taking into account both biological factors (soft tissue irritation and tooth sensitivity), the incidence of discomfort during bleaching was higher for the patients who underwent bleaching with bleaching strips.
Other clinical studies showed that the percentage of tooth sensitivity and soft tissue irritation are proportional to the concentration of HP in bleaching strips, the contact time with tooth and soft tissues, and the total treatment time (Kugel et al. 2011; Donly et al. 2010). According to the data reported by Swift et al. (2009), about 80 % of soft tissue irritation cases occurred after long treatment periods in patients who used bleaching strips. Lucier et al. (2013) evaluated the toxic effect of bleaching strips containing 6–14 % HP on the gingival epithelium in vitro in a three-dimensional culture model. The authors observed that the application of the bleaching strips for 30 min resulted in changes in tissue morphology that were associated with the apoptotic death of cells in all epithelial layers, inducing keratinocyte proliferation and increased expression levels of proinflammatory cytokines. These effects were proportional to the HP concentration.
The presence of cracks, enamel craze lines, exposed dentin, caries lesions, wear facets, noncarious cervical lesions, restorations with marginal gaps, gingival recession, gingivitis, and periodontal disease can influence the extent of the harmful effects of bleaching products on oral and pulpal tissues.
However, the negative effects caused by bleaching agents used in these specific clinical situations have been rarely studied. By contrast, a number of studies have evaluated the aesthetic effectiveness of bleaching products and techniques. Thus, the dentist should carry out a careful clinical exam prior to starting the bleaching treatment in order to determine the ideal treatment for each particular case. As discussed above, the use of products containing HP for tooth bleaching without professional supervision represents a health risk to the population generally unaware of the factors involved with the use of such products and the conditions of their oral health. Regulatory bodies in Brazil (ANVISA 2015) and in the European Union (European Commission Scientific Committee on Consumer Products 2007; European Commission Scientific Committee on Consumer Safety 2010) have already restricted the commercialization of sodium perborate and hydrogen peroxide-based bleaching products in order to protect the population from the risks of using bleaching agents without professional supervision. However, in several countries, including the United States, such products are still accessible over-the-counter to the general population and have great economic impact owing to the strong aesthetic appeal involved (Demarco et al. 2009). Nevertheless, according to the Food and Drug Administration (2003), hydrogen peroxide is safe at concentrations of up to 3%, but there are insufficient data available to permit final classification of its effectiveness at 1.5–3 % concentrations for long-term OTC use in the mouth.
4.2.3 In-Office Bleaching
It is well established that once the in-office technique is decided upon, all oral soft tissues, as well as the face and eyes of patients, must be protected from accidental contact with the bleaching products. Clinical studies in which qualified practitioners performed the entire bleaching procedure reported a percentage of 0–4 % of patients with mild to moderate soft tissue irritation, irrespective of the HP concentration used (Marson et al. 2008; Ward and Felix 2012). This result is expected, as the placement of a suitable gingival barrier with flowable light-cured resin effectively prevents the contact of the bleaching gel with the gingival and periodontal tissues. However, the gingival barrier must be created carefully, and it must extend not only to the cervical region of the teeth to be bleached but also to the adjacent soft tissues in order to prevent inadvertent contact with the bleaching product (please refer to video “In-Office Whitening”). Lip retractors and labial and lingual protectors should also be used with constant suction. Figure 4.3 demonstrates the correct use of gingival barriers and intraoral protective equipment in the in-office bleaching. This equipment will prevent the contact of the bleaching agent with other oral tissues caused by inadvertent movements of the patient. At the end of the application period, the gel must be carefully aspirated from the tooth surface, followed by washing with simultaneous suction, in order to prevent the flow of highly concentrated product to the oral cavity and the swallowing of product residue by the patient (Fig. s4.3).
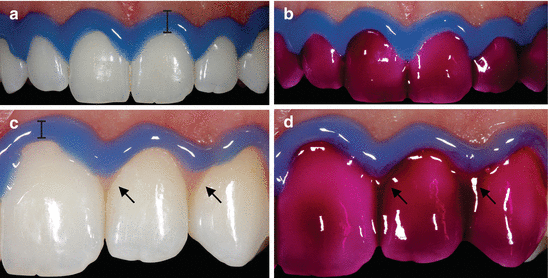

Fig. 4.3
(a) Correct use of the gingival barrier, carefully positioned in the cervical region of the enamel, covering interdental regions and a considerable portion of the marginal gingiva. (b) Application of bleaching gel in ideal conditions, minimizing the possibility of accidents with the product. (c) Incorrect application of the gingival barrier, which does not adequately extend to the tooth surface, only extending slightly into the soft tissue (arrow) and gingival papillae. (d) A typical accident during the bleaching procedure – the bleaching gel contacts the gingival tissue (arrow). This is a placebo gel without HP, just for illustration purposes
However, quite often, gingival barriers are positioned inappropriately or are moved during the procedure, allowing direct contact of highly concentrated and thus toxic peroxides with the adjacent gingival tissue. When such accidents occur, the mucosa becomes temporarily whitened and is likely to return to clinical normality after the application of a neutralizing agent and rehydration. These effects may be observed in Figs. 4.4 and 4.5.
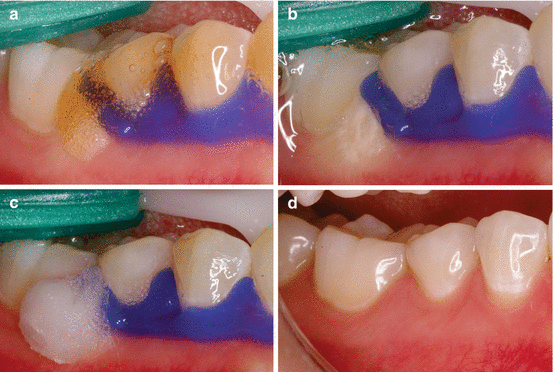
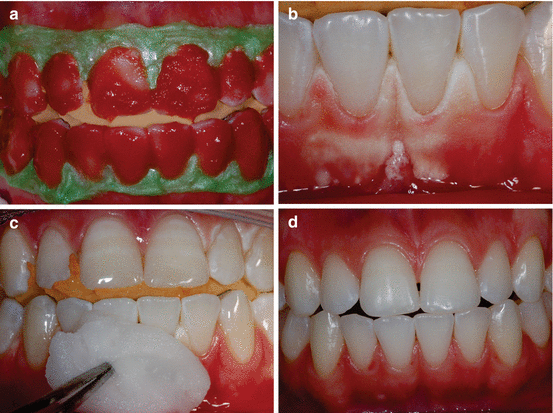

Fig. 4.4
(a) Seepage of 35 % HP bleaching gel to the region that is not protected with the gingival barrier. (b) Clinical characteristic of the gingival tissue immediately after contact with the bleaching gel. (c) Application of a 10 % sodium bicarbonate neutralizing agent. (d) Clinical characteristic of the gingival tissue 7 days after the incident

Fig. 4.5
(a) Mandibular incisors submitted to in-office bleaching treatment with 38 % HP. (b) Either the application of the gingival barrier in an excessively humid operative field or the extended time that the gel was in contact with the teeth may have caused alterations in the gel’s thixotropic characteristics and lead to a leakage of gel, causing extensive damage to the soft tissue. (c) Application of neutralizing product based on sodium bicarbonate. (d) Clinical aspect 45 min after the incident
Figure 4.6 depicts a clinical case in which the gingival papillae retracted and became erythematous after the at-home part of a “jump start” technique procedure (described in Chap. 6). During prolonged contact with the oral mucosa, significant epithelial alteration associated with acute inflammation of the underlying connective tissue may occur. These pathological changes generated by incorrectly performing the bleaching procedure may cause discomfort of the patient. The severity of the damage to the buccal mucosa can be directly related to the concentration of HP present and/or released by the bleaching product, its pH, and the time of contact of the gel with the tissue. In an ongoing in vivo study by our research group, the oral mucosa of rats is exposed to the application of different bleaching gels for 30 min. Then, biopsy samples of damaged tissues treated or untreated with a neutralizing agent (such as sodium bicarbonate) are obtained and processed for microscopic analysis of tissue response. Preliminary analysis of histological sections stained with hematoxylin and eosin revealed that the extent of tissue changes varied according to the bleaching product and that treating the damaged mucosa with a neutralizing agent reduces the extent of damage caused by the bleaching gels, particularly those with HP concentrations greater than 15 % (Fig. 4.7).
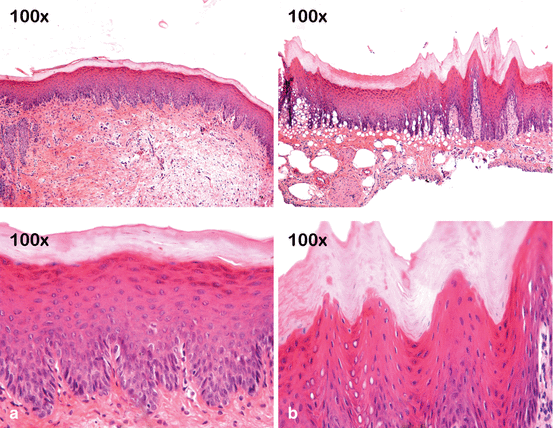
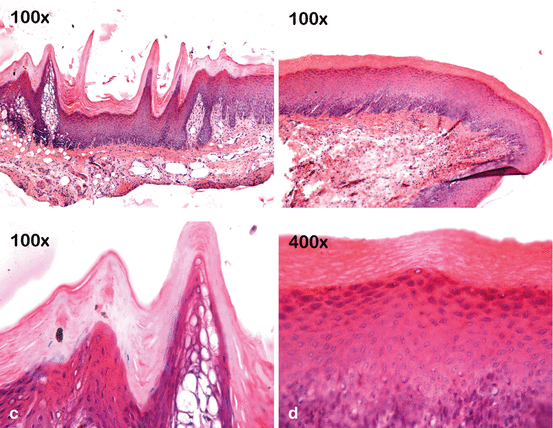
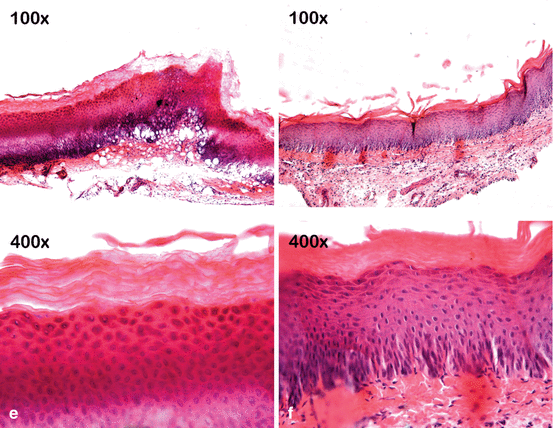

Fig. 4.6
Effect of 10 % CP on the gingival papilla region after the at-home component of a “jump start” technique procedure. An allergic reaction to a component of the bleaching product may have occurred since no tray compression was observed in the papilla region. (a) Pretreatment view. (b) After removing the bleaching agent and gingival barrier, retraction in the gingival papilla associated with an erythematous surface is observed in the region between teeth #6 (13) and #9 (21). (c) Clinical aspect after 7 days



Fig. 4.7
Bleaching gels are applied to the buccal mucosa of rats for 30 min, and then the injured tissue is treated or untreated with a neutralizing agent (sodium bicarbonate). The mucosa exposed to 10 % CP gel does not show any noticeable change in the epithelium and underlying connective tissue (a, d). However, the epithelium treated with gels containing 15% (b, e) or 35 % (c, f) HP shows numerous fingerlike papillae, acanthosis, and large areas of cell vacuolation. Intense inflammation associated with cell hydropic degeneration and extensive areas of edema can be observed in the underlying connective tissue. However, these tissue changes appeared less intense when the mucosa of the animals exposed to these gels with high HP concentrations were subsequently treated with a neutralizing agent
4.3 Effect on Oral Hard Tissues
4.3.1 Change in Color of Tooth Structure
Tooth bleaching has been the first choice of treatment of intrinsic pigmentation of tooth structure (Williams et al. 1992; Perdigão 2010). The bleaching process is believed to occur via the action of the low-molecular-weight HP, which easily diffuses through the enamel and dentin, releasing reactive oxygen species that effectively promote the oxidation of the organic substrate present in the tooth structure. As a result, dental pigmentation molecules become simpler or are eliminated. Although the traditional in-office bleaching technique (30–40 % HP, applied for 30–60 min) provides highly satisfactory cosmetic results in a short period, the biological effects are currently controversial because of the scientific evidence proving that this therapy can cause irreversible damage to the pulp-dentin complex.
The intense tooth sensitivity in patients treated with in-office bleaching causes great discomfort to patients, which has led researchers to reassess the concepts used in the last decades.
Our research group has evaluated some parameters for the application of at-home and in-office bleaching techniques, with the aim of finding more effective and more biocompatible bleaching techniques. These parameters include the following: (1) the need to irradiate the in-office bleaching product with a light source; (2) HP concentration in bleaching gels; (3) the contact time of the product with the dental surface; (4) the need for reapplication of the product on the tooth surface during the same clinical session; (5) the composition of the bleaching gel (CP or HP); (6) combined use of at-home and in-office bleaching techniques; and (7) acid etching of the enamel prior to bleaching.
The irradiation of in-office bleaching agents with light sources has had a strong commercial appeal in recent decades. It has been widely used in dental offices to (supposedly) accelerate the bleaching procedure, a technique known as power bleaching. The action mechanism proposed for irradiation with light is based on thermocatalysis, resulting in a twofold increase in HP decomposition with a temperature increase of 10 °C (Buchalla and Attin 2007). However, the real benefits of light-activating bleaching products remain controversial in the literature. According to in vivo and in vitro studies conducted by our group, irradiation of the 35 % HP bleaching gel with halogen lamps (20–40 s/application), and LED (60 s/application) or LED/laser sources (3 min/application), did not promote a significant increase in the bleaching effect after the first bleaching session to 1–6 months after bleaching. Patients who underwent bleaching with light irradiation reported a longer duration and greater intensity of tooth sensitivity (Almeida et al. 2012; Simões et al. 2015).
Based on these findings, the use of traditional in-office gels in combination with light sources should be eliminated from everyday practice.
In relation to the HP concentration used in the in-office technique, in vitro studies that used ultraviolet reflection spectrophotometers showed that the color change was saturated after three or four sessions when 35 % HP gels were used, with about 50–60 % of the total color change obtained after the first bleaching session (Briso et al. 2010b; Soares et al. 2014a; de Almeida et al. 2015b). By using nonstained specimens with external pigments, previous authors have observed that a 20 % HP gel resulted in the same bleaching outcome as a gel with 35 % HP; this means that about 60 % of the color change occurred after the first bleaching session, based on the similar color change pattern observed after the second and third sessions (de Almeida et al. 2015b). When specimens stained with black tea (yellow pigment) were used, a 17.5 % HP gel promoted a gradual color change in the tooth structure. While a more concentrated gel (35 % HP) caused 50 % of the color change after the first session, a 17.5 % HP gel promoted about 36.5 % color change, with the bleaching effect being intensified gradually throughout the sessions so that no difference with the traditional 35 % HP protocol was observed at the end of four sessions (Soares et al. 2014a). It is noteworthy that these results obtained for both 35 % HP gel and for 17.5 % HP gel were measured for the same duration of contact with the tooth structure (45 min).
The advantage of using in-office bleaching materials of lower concentration is based on the fact that these products minimize HP diffusion over the tooth structure by about 60 %, which has a positive biological effect on pulp cells and confers less risk to the oral mucosa.
Our data is in agreement with the results of Sulieman et al. (2004). These authors observed that the bleaching efficacy was proportional to the concentration of the bleaching agent applied on teeth discolored with black tea. It took 2, 4, 7, and 12 applications for the gels containing 25 %, 15 %, 10 %, and 5 % HP, respectively, to reach the same bleaching effect observed after a single application of the gel containing 35 % HP. Thus, gels with reduced HP concentration can reach the same bleaching standard attained with more commonly used higher HP concentrations. However, the effects of lower HP concentrations are more gradual and depend on intensity of the stains.
Similar results were obtained for the at-home bleaching technique. After treatment, gels with 16 % and 20 % CP were observed to have the same bleaching potential as a 10 % CP gel, with the latter resulting in lower tooth sensitivity and lower incidence of soft tissue irritation (Meireles et al. 2010; Basting et al. 2012). Regarding the presentation form, we observed that home bleaching gels with 10 % CP have the same bleaching potential as gels with 6 % HP when applied using the same treatment regimen (1.5 h daily for 3 weeks). However, the HP-based gel resulted in HP diffusion into the tooth structure at about 50 % greater intensity. Furthermore, the application of the product with 10 % CP for 1.5–3 h resulted in the same aesthetic effect after 7, 14, and 21 days of treatment; and the shorter the contact time, the lower the HP trans-amelo-dentinal diffusion (de Almeida et al. 2015a). Home treatment with gels containing 10 % CP, applied for 3–4 h a day for 3 weeks in a custom-fitted tray, had the same bleaching potential as traditional in-office bleaching (Briso et al. 2010b; Almeida et al. 2012; Basting et al. 2012).
Figure 4.8 is a graph developed from data observed in our laboratory and clinical trials. The at-home bleaching technique using either 10–16 % CP or 3–7 % HP in a custom-fitted tray versus the in-office technique (20–40 % HP) most often provides similar results at the end of the third week of treatment, reaching the chromatic saturation in most of the cases within this period. We have also found that the combination of at-home and in-office techniques (jump start) provides a faster color change at the beginning of treatment, which makes this an interesting option to accelerate the aesthetic result. The association of in-office bleaching sessions with low HP concentrations (15–20 %), with daily applications of bleaching gels with 10 % CP over a short period (1.5 h daily), following the supervised home bleaching technique (scalloped custom-fitted tray with no reservoirs), presents itself as an attractive alternative to accelerate the aesthetic result using more biologically compatible bleaching techniques. However, practitioners should be aware that the indication of the at-home technique should be based on a detailed initial clinical examination to avoid the application of the material in areas that may increase the toxic potential of this therapy to the oral tissues. These precautions will be discussed later in this chapter.
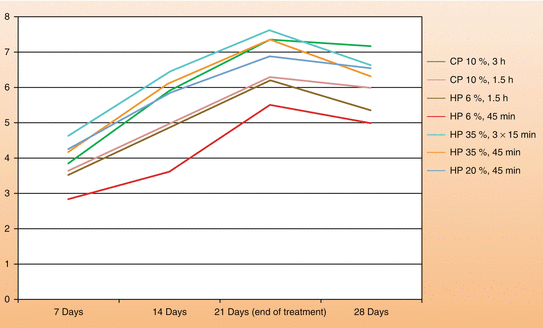

Fig. 4.8
Color change (Delta E), according to bleaching posology employed and treatment time
The need for successive reapplication of the bleaching product during the same clinical session has also been questioned. In a recent study conducted by our group, we observed that bleaching gels with 35–38 % HP retain about 86 % of the initial concentration of HP after 45 min of contact with the tooth structure (Marson et al. 2015).
These results demonstrate that reapplication of the bleaching product during in-office bleaching is not necessary.
In the study of de Almeida et al. (2015b), a 35 % HP gel resulted in the same bleaching outcome when applied once for 45 min or three times for 15 min each. Similarly, Soares et al. (2014a) found that the continuous application of a 17.5 % HP gel for 15 min or reapplication of the product three times for 5 min each resulted in the same aesthetic effects after six whitening sessions. The application of the in-office bleaching product on the tooth structure over reduced periods of time promotes gradual and effective bleaching when 35 % HP bleaching gels were tested (Soares et al. 2014a). However, application of gels with low HP concentrations on the tooth structure over short periods (5–15 min) resulted in a limited lightening effect, even after six bleaching sessions (Soares et al. 2014a).
Finally, acid etching of the tooth structure prior to in-office bleaching has been indicated in order to increase the effectiveness of this clinical procedure. In an experiment conducted recently by our research group, etching the enamel with 37 % phosphoric acid for 20 s immediately prior to application of 35 % HP bleaching gel (three applications of 15 min each) did not result in a significant increase in bleaching effectiveness and did not interfere with HP diffusion over the tooth structure. Thus, as enamel acid etching with phosphoric acid changes the mineral structure of the enamel and removes hydroxyapatite without benefiting the bleaching outcome, it should not be used with in-office whitening.
4.3.2 Microabrasion and Tooth Bleaching
In some cases, stains can still be observed on the enamel after completion of the bleaching treatment. While there may be several types of stains, we found that these usually have well-defined contours and are whitish. Some of these stains can be transient and become imperceptible with the color stabilization and rehydration of the dental element after bleaching. Often, these white spots already existed but only became apparent after the color change produced by the treatment. On the other hand, yellow teeth with enamel whitish stains may be candidates for tooth whitening to have the stains attenuated.
Considering the texture or color changes of the surface layers of enamel, enamel microabrasion using acidic and/or abrasive agents has been suggested as an excellent alternative to improve the appearance of the teeth (Croll 1997) by camouflaging the white spots when they are not very deep (Chap. 9). Although some studies (Paic et al. 2008; Rodrigues et al. 2013) showed that the wear of the tooth surface after this procedure is minimal, it is necessary to consider that these changes can reach different depths. Additionally, the aprismatic enamel layer may also be affected during removal, in addition to the removal by enamel etching by hydrochloric acid in the microabrasive material. These changes may substantially alter the permeability of dental hard tissues. This issue has concerned some researchers, especially when bleaching with 35 % HP is carried out immediately after the microabrasion procedure, as the HP diffusion over the tooth structure in these conditions is about 20 % higher (Briso et al. 2014a). This increase in HP diffusion may decrease the safety of the procedure considerably.
Thus, in most cases, the microabrasive treatment is complemented with bleaching because of the yellowish color of the teeth after erosion of the enamel. However, for convenience, aesthetic rehabilitation is initiated by performing the bleaching treatment, which may be sufficient to make the intrinsic enamel stains partially or totally imperceptible, as previously described. If the enamel microabrasion is still deemed necessary, teeth will invariably present a more yellowish appearance due to enamel removal and consequent proximity to dentin. In these cases, a waiting period of 7 days is recommended before at-home bleaching is initiated with low-concentration bleaching products.
4.3.3 Change in Hardness/Susceptibility to Caries/Demineralization/Importance of Saliva
The effect of bleaching agents on dental enamel has been extensively studied in the literature (Kwon et al. 2002; Spalding et al. 2003; Cavalli et al. 2004; Faraoni-Romano et al. 2008; Forner et al. 2009). Morphological changes, increased surface porosity, exposure of prisms, reduced organic content, change in the calcium/phosphate proportion, and reduced microhardness, are the main changes that occur in enamel upon bleaching. These changes depend on the contact time of the gel with the dental substrate, the CP/HP concentration in the product, and the pH of the product during its use.
Soares et al. (2013b) showed that 16 % CP gel resulted in the formation of deeper pores on the enamel surface compared to those formed with 10 % CP, along with a more pronounced loss of calcium and phosphorus. Taking into account that the only variable was the CP concentration, being all the other parameters standardized (pH of the bleaching gel, the interval between applications, and the total treatment time), the concentration of the bleaching gel was responsible for the more pronounced changes observed when a 16 % CP gel was used. Current literature also demonstrates that the use of high HP concentrations induces more pronounced alterations in the ultimate tensile strength of human enamel accompanied by changes in the enamel’s internal micromorphology and some intraprismatic material loss (da Silva et al. 2005) (more information in Chap. 6).
As the pH of at-home CP bleaching gels ranges from 5.6 to 7.3 and the urea released during the degradation of CP increases the pH within 15 min, the original pH of these gels is unlikely to have any association with structural changes in the enamel, even with prolonged contact time with the tooth surface. Thus, the pores are formed on the enamel surface after bleaching as a result of the disruption of enamel protein matrix and subsequent loss of the crystalline material surrounded by this matrix. This hypothesis derives from the observation in several studies that enamel dissolution occurs heterogeneously, with areas of erosion interleaved with areas of intact enamel (Kwon et al. 2002; Spalding et al. 2003; Cavalli et al. 2004). As the distribution of proteins and other organic materials are uneven on the enamel surface, the defects observed after bleaching occur heterogeneously (Kwon et al. 2002). Other studies have demonstrated that the dissolution occurs primarily in the interprismatic regions and in the enamel hypomineralization areas, which are the regions with the greatest amount of organic material (Spalding et al. 2003; Cavalli et al. 2004).
When gels with high HP concentration were used for in-office whitening, the morphological changes on the enamel surface were significant enough to be observed even after a single application of the product on enamel, increasing the density of pits and different degrees of porosity (Kwon et al. 2002; Spalding et al. 2003). These changes may have been caused synergistically by the oxidative effect of HP and its acidic pH. Although the average pH of in-office bleaching products is around 6.5, many gels have a pH between 3.6 and 5.0 (Price et al. 2000), which are pH values below the critical pH for enamel dissolution (5.5). Recent studies have shown that the pH of bleaching product has a direct relationship with the roughness of tooth enamel after bleaching and that the pH of bleaching agents tends to decrease with increased contact time with the tooth structure (Trentino et al. 2015; Abe et al. 2016).
Despite the various morphological changes observed on the enamel surface, studies have shown that these changes are mild to moderate. However, the contact of bleaching products with dentin can cause more severe changes. Reduction in wear resistance (Faraoni-Romano et al. 2009), decreased hardness (Faraoni-Romano et al. 2008; Forner et al. 2009), and increased surface roughness (Faraoni-Romano et al. 2008) have been described to be more pronounced in enamel. These findings may be explained by the specific composition of dentin, which has a greater organic content than enamel. Additionally, dentin has increased susceptibility to the HP oxidative action and acidic pH of the bleaching gels, as the critical pH value for the dentinal dissolution is between 6.2 and 6.7 (Faraoni-Romano et al. 2009). Therefore, the contact of bleaching agents with exposed dentin areas is highly contraindicated.
As the changes in the enamel are considered subtle, it remains a challenge how to extrapolate these results to the in vivo situation, where factors such as saliva and the presence of fluoride may act to remineralize tooth structure (Kwon et al. 2002). Studies that performed bleaching in situ (Rodrigues et al. 2005; Faraoni-Romano et al. 2009) or applied human or artificial saliva to specimens in between the bleaching steps (Spalding et al. 2003; Faraoni-Romano et al. 2008; Sasaki et al. 2009) showed insignificant changes in the enamel, which is attributable to the remineralizing action of saliva. Sasaki et al. (2009) also demonstrated that the storage in artificial saliva of specimens bleached for 14 days resulted in a significant increase in microhardness. In their study, Spalding et al. (2003) observed under scanning electron microscopy that bleaching with 35 % HP followed by immersion in human saliva for 1 week resulted in the formation of a granular blanket on the enamel surface, which was probably due to remineralization process by saliva. Soares et al. (2013a) observed that the use of solutions with 0.2 and 0.05 % sodium fluoride for 1 min after each application of the bleaching gel prevented the structural changes observed in the enamel when gels containing 10 and 16 % CP were used. Kemaloğlu et al. (2014) also demonstrated that fluoridated solutions (2.1 % sodium fluoride) significantly prevent mineral loss in enamel subjected to 38 % HP bleaching gels.
Although these changes tend to reverse when in contact with saliva and fluoride, the use of peroxides in demineralized areas may worsen existing conditions. In this context, during clinical examination, the practitioner must pay attention to the presence of incipient carious lesions that may have become wider due to the bleaching treatment. In a recent study conducted by our group, we found that the application of a 35 % HP gel (three times for 15 min) on specimens with demineralized enamel to simulate incipient lesion caries resulted in a more intense HP diffusion over the tooth structure than that observed in healthy and bleached specimens. In the same study, we found a greater reducing effect on enamel microhardness when demineralized specimens were bleached, wherein the bleaching increased the depth of demineralization of incipient caries lesions. The surface and subsurface morphology were also more heavily affected in the previously demineralized enamel subjected to bleaching (Briso et al. 2015).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


