
7

Classic Approaches to Sialoendoscopy for Treatment of Sialolithiasis
Obstructive sialadenitis, with or without sialolithiasis, represents the main inflammatory disorder of the major salivary glands. The diagnosis and treatment of obstructions and inflammations of these glands can be problematic due to the limitations of standard imaging techniques. Satisfactory treatment depends on our ability to reach a precise diagnosis and, in the case of sialoliths, to accurately locate the obstruction. Until recently many of these glands required complete removal under general anesthesia.
Sialolithiasis is a common finding, accounting for 50% of major salivary gland disease.1,2 The submandibular gland is the most prone to sialolithiasis. In various studies it was found that ~80% of all sialolithiasis cases are in the submandibular glands, 19% occur in the parotid gland, and ~1% are found in the sublingual gland. Sialolithiasis is most often found in adults, but it may be diagnosed in children.3
Sialoliths may vary in size, shape, texture, and consistency. They may occur as a solitary stone or as multiple stones. Bilateral submandibular stones are a rare condition (5% of submandibular sialolithiasis cases). Sialolithiasis of submandibular and parotid gland together has not been reported in the literature. The amount of symptomatic and nonsymptomatic sialolithiasis cases is 1% of the population, found in autopsy material.4
The symptomatic group of patients admitted to the hospital each year has been estimated as 57 cases per million per annum in the British population, representing 3420 patients per annum.1 If this incidence is applied to the European or the American population (300 million), then ~17,100 patients per annum will require hospital treatment for sialolithiasis and its complication sialoadenitis. These data do not include patients who were treated as ambulatory (outpatient) cases.
There is a male preponderance,5 and the peak incidence is between the ages of 30 and 60.5 Sialoliths grow by deposition and range in size from 0.1 to 30 mm.6 Presentation is typically with a painful swelling of the gland at meal times, when the obstruction caused by the calculus becomes most acute.7
During the past decade, with the introduction of salivary gland endoscopy there has been a major step forward, not only in providing an accurate means of diagnosing and locating intraductal obstructions, but also in permitting minimally invasive surgical treatment that can successfully manage those blockages that are not accessible intraorally.8–20
 Clinical Presentation
Clinical Presentation
See Chapter 5 for a full discussion of the clinical presentation of sialoliths.
 Diagnostic Methods
Diagnostic Methods
Clinical Evaluation
Visual scanning of submandibular, preauricular, and postauricular regions is the first step in assessing swelling and erythema (see Chapter 5). This is followed by intraoral examination. Surgical magnification loops (2.5–3.5) are very useful in improving visualization of the orifice of Wharton’s and Stensen’s ducts. The orifice may be red and edematous and appear as a papilla. Plaques or whitish secretions from the duct may represent frank infection. Sometimes a small stone can be found in the orifice; occasionally, the white-yellow color of a stone can be seen through the translucent mucosa. Bimanual palpation is particularly important when examining the submandibular gland and duct. It helps to differentiate the gland from adjacent lymph nodes, inferior to the gland, and to ascertain the presence of any firm mass in the take-off of Wharton’s duct from the hilum of the gland.
For the parotid gland, manual palpation allows the surgeon to determine the consistency of the gland. One should also massage the gland to milk and inspect the saliva.
Salivary Imaging
Although there are a variety of newly available imaging methods, in this section we focus on those techniques most suitable for patients suffering from salivary gland obstructions (See also Chapter 2). The most effective imaging methods for inflammatory conditions of the submandibular and parotid glands are plain x-rays (occlusal, occlusal oblique, panoramic), sialography, ultrasound, and computed tomography (CT). Scintigraphy will be included in this chapter because of its unique ability to evaluate the gland function. Sialoendoscopy is a newly developed technique that is useful for imaging and treatment. It will be discussed separately.
Plain X-ray
Traditionally, plain radiographs are often used as a simple first-line investigation. Occlusal, occlusal oblique, and panoramic x-rays are excellent for ruling out any calcification in the submandibular region (Fig. 7–1). These will not demonstrate radiolucent calculi, which account for 20 to 43% of submandibular stones.21,22 For parotid stones, panorex and anteroposterior views directed to the parotid region are recommended. The practitioner has to remember that plain x-rays have minimal value in parotid stones because of the amount of radiolucent stones (60–70%). The plain x-ray gives no information on the condition of the affected gland. It is therefore necessary to supplement or supplant plain radiography with another diagnostic modality.
Sialography
Sialography is one of the oldest salivary gland imaging techniques. The first contrast agents used in the early twentieth century were pure mercury. The dye that he used was pure mercury. Nowadays we have better dye options. Although there is a need to penetrate to the ductal system with a catheter through the ductal papilla, it is the only method that can give the possibility to examine the ductal system with reasonable cost. Reducing the discomfort during sialography may be achieved by applying topical anesthesia to duct papilla and ~or by lavaging the gland through the orifice with 2% lidocaine prior to the injection of the water-soluble dye.
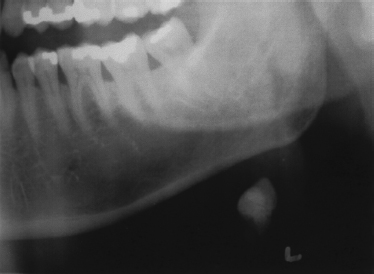
FIGURE 7-1 Panoramic dental x-ray, demonstrating large sialolith in the left submandibular gland.
Sialography provides images of the morphology of the ductal system and allows the diagnosis of strictures, dilatations, and filling defects. This technique also provides information on glandular function (Fig. 7–2A).
Ultrasound
High-resolution ultrasound is a good imaging method to assess the salivary glands. It is noninvasive, and there is no associated discomfort. It is useful to distinguish the submandibular gland from surrounding lymph nodes and to locate calculi. The portion of Wharton’s duct that leads from the hilum of the gland toward the floor of the mouth, precisely after the penetration of the mylohyoid muscle, is difficult to identify.23 Calculi detection rates vary between 63 and 94%16 and are close to those for sialography.24 Ultrasound is able to detect radiolucent stones even though the acoustic shadow is not as marked. The distal portion of the submandibular and parotid ducts can be difficult to visualize using extraoral ultrasound.25 However, small, high-frequency intraoral probes are now available that overcome this limitation26 (Figs. 7–2B, Figs. 7–3).
Computed Tomography
CT scan is especially useful for evaluating inflammatory conditions of the submandibular and parotid glands. Sialoliths are readily identified on CT imaging. The standard images should be 1 mm cuts with three-dimensional reconstruction. In this way the glands and ducts can be visualized in all planes, and stones are less likely to be missed. The parotid gland and duct are well demonstrated by CT. Another advantage is the possibility to diagnose and locate intraparenchymal stones and calcifications that are not connected to the gland (phleboliths, tonsiliths, calcifications in the lymph nodes, etc.) (Fig. 7–4).
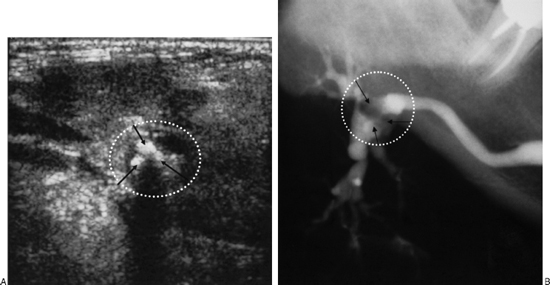
FIGURE 7-2 (A) Ultrasound and (B) sialogram of the right submandibular region of patient suffering from multiple swellings. Three stones are demonstrated in the sialogram and in the ultrasound (arrows). The stones appeared as hyperechogenic lesions with acoustic shadow.
Scintigraphy
In contrast to ultrasound, which depicts architecture, radioisotope imaging of the salivary glands gives some measure of the secretory function and allows comparison between the major glands. The assessment of salivary gland function using a bolus intravenous injection of technetium Tc 99m pertechnetate is easy to perform, reproducible, and well tolerated by the patient.27 It enables examination of the parenchymal function and excretion rate of the salivary gland and has the further advantage of a short half-life and low radiation dose.27
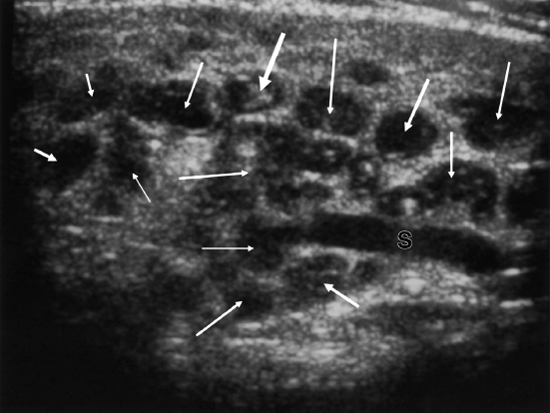
FIGURE 7-3 Ultrasound of parotid gland with multiple hypoechogenic sialectases (arrows) and dilated Stensen’s duct (S).
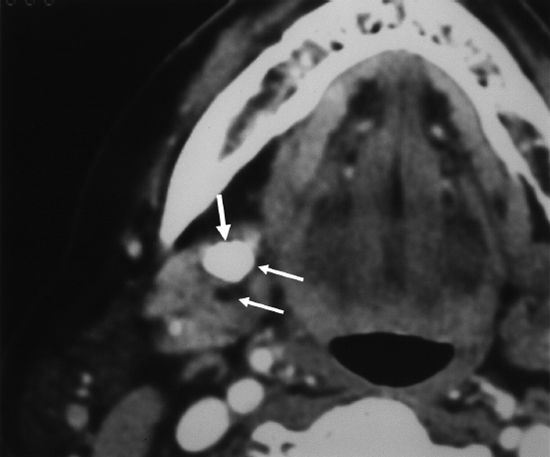
FIGURE 7-4 Computed tomography scan of submandibular gland with stone. The sialolith is marked with arrows.
 Surgical Procedures
Surgical Procedures
This section is problematic because of the enormous and rapid development of methods and technology in recent years. As in other fields of surgery, traditional and more aggressive techniques are being replaced by organ-preserving methods with the help of minimally invasive techniques. The reader needs to be familiar with all techniques. This section includes two parts: traditional and modern approaches.
Traditional Approaches to Submandibular Sialolithiasis
Traditionally, sialoliths in the submandibular duct and gland were divided into two groups: (1) stones that can be removed through intraoral sialolithotomy approaches, including stones up to the first molar tooth, which can be palpated; and (2) stones that cannot be removed from the intraoral approach and require sialadenectomy, including stones posterior to the first molar region, or stones in the middle part of the Wharton’s duct that cannot be palpated intraorally.
Intraoral Sialolithotomy
The first step is to locate the stone exactly. This technique is useful only in stones in the anterior and middle part of Wharton’s duct up to the first molar tooth. Effectively, only stones that can be palpated easily from the intraoral region are candidates for this technique.
Following administration of local anesthesia, two sutures of 3.0 silk are placed posterior to the location of the stone. The aims of this step are to isolate the stone and to prevent movement of the stone to the inner part of the duct or hilum of the gland. The next step is to cut the mucosa above the stone directly on the stone, which can be done with a cold blade, electrosurgery, or CO2 laser.28 The advantages of the CO2 laser are the hemostatic effect and the easy identification of the stone. The contact between the stone and the laser beam creates a spark that can be easily identified. Following incision of the mucosa and the duct, the stone is exposed and extracted with dental curettes. Following the extraction of the stone, the silk sutures are removed. Milking the gland allows discharge of plaques and saliva and possibly additional stones. Interrupted 4.0 Vicryl sutures are placed to connect the ductal layer to the oral mucosa. The patient is encouraged to massage the gland and is treated postoperatively with oral antibiotics for 7 days.
The same procedure of sialolithotomy has been described for the more posterior region. The author is strongly opposed to this technique because of the high risk of injury to the lingual nerve and the possibility of severe bleeding from lingual vessels. The technique of extracting such stones is described in the section Modern Endoscopic Approaches to Sialolithiasis.
Submandibular Sialadenectomy
See Chapter 15 for a full discussion of submandibular sialadenectomy.
Traditional Approaches to Parotid Sialolithiasis
Traditionally, sialoliths in the parotid duct and gland were divided into two groups. (1) stones that can be removed through an intraoral sialolithotomy approach, including stones up to the curvature of Stensen’s duct above the masseter muscle; and (2) stones that cannot be removed with an intraoral approach, requiring extirpation of the parotid gland. This would include stones posterior to the curvature of the duct.
Intaoral Sialolithotomy
The first step is to locate the stone exactly. This technique is useful only in stones in the anterior part of Stensen’s duct anatomically demarcated by the curvature of the duct above the masseter muscle as the duct penetrates the buccinator muscle. Following local anesthesia around the papilla of Stensen’s duct, a lacrimal probe is advanced until it reaches the stone. A hemostat holds the papilla and the probe to ensure safe tract to the stone. An elliptical incision around the papilla and the probe is indicated preferably with a CO2 laser. Blunt and sharp dissection is performed around the duct up to the stone location. The ductal layer above the stone is incised and the stone removed with dental curettes. Following the sialolithotomy, massage of the gland allows release of plaque and saliva. The ductal layer is sutured with several 4.0 Vicryl sutures to the oral mucosa to promote a patent duct.
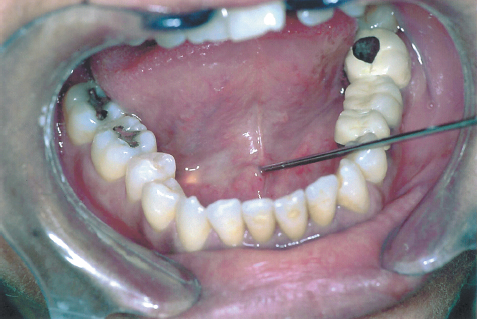
FIGURE 7-5 Introduction of 1.3 mm diagnostic unit through the orifice of the Wharton’s duct (following dilatation) into the gland. Note the transillumination effect.
Superficial and Total Parotidectomy
See Chapter 15 for a full discussion of superficial and total parotidectomy. The difference between removal of the parotid gland with stone and benign tumor is the condition of the gland. Scar tissue, inflammation, and fibrosis inside the gland and around Stensen’s duct make the operation difficult and heighten the risk for facial nerve damage and salivary fistulae.29–31
Modern Endoscopic Approaches to Sialolithiasis
In the past decade, the advent of salivary gland endoscopy has brought us a major step forward, not only in that the novel techniques provide an accurate means of diagnosing and locating intraductal obstructions, but also that they permit minimally invasive surgical treatment that can successfully manage blockages not amenable to an intraoral approach.8–20
In 1997 we (Nahlieli and Baruchin11) reported on our experience with the use of a mini rigid endoscope to perform sialoendoscopies on 46 major salivary glands. To date we have successfully managed 892 patients with these endoscopic techniques.
Indications
The indications for sialoendoscopy are the following:
- For diagnostic purposes, recurrent episodes of major salivary gland swelling without obvious cause
- Sialolithotomy: removal of deeply located stones (posterior portion of Wharton’s duct (“comma area,” because of its proximity to the lingual nerve) or stones in Stensen’s duct posterior to curvature of the duct above the masseter muscle
- Exploration of the ductal system following calculi removal from the anterior or middle part of the submandibular or parotid ducts
- Strictures or kinks of the salivary ductal system
- Treatment of submandibular and parotid sialadenitis
- Pediatric inflammatory and obstructive pathology
Absolute Contraindication
Acute sialadenitis is an absolute contraindication for sialolithiasis.
Pre-endoscopic Assessment
Following the clinical evaluation, plain x-rays, which include panorex, occlusal, occlusal oblique, sialogram, and ultrasound, are recommended. In the case of a parotid stone in the middle or posterior parts, a CT scan is indicated.
Introduction of the Sialoendoscope
To determine the feasibility of entering the ductal lumen by use of sialoendoscopy, the size of the duct is measured by sialography and ultrasound imaging. Sialography is used for mapping the ductal system for possible variations and assessment of its estimated dilatation capacity.
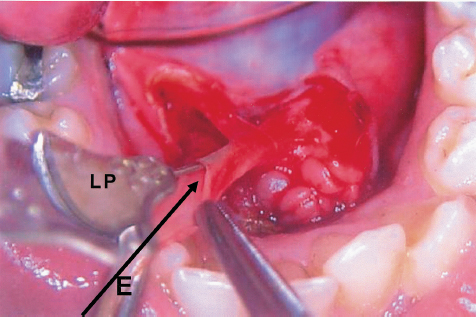
FIGURE 7-6 Exploration of the Wharton’s duct. Note the diameter of the duct, sufficient for insertion of the surgical endoscope for interventional sialoendoscopy. Lacrimal probe (LP) is in the duct for correct location. Note the position of the endoscope (E) for accurate insertion.
There are four possible methods for introducing the endoscope into the ductal lumen: (1) introducing the exploration unit (1.3 mm) through the natural orifice of the duct; sometimes there is a need for dilatation, which can be done with lacrimal probes (Fig. 7–5); (2) through a papillotomy procedure, performed with a CO2 laser immediately posterior to the orifice of the duct, thus enlarging the opening; (3) through ductal exploration (“ductal cutdown”), which involves surgical dissection and exposure of the anterior portion of the duct with a microsurgical technique. The duct is then incised longitudinally to allow the intraluminal insertion of the endoscope. If there are any difficulties in introducing the endoscope in the anterior part (e. g., stricture, too narrow ductal lumen), it may be necessary to expose the duct more posteriorly to arrive at a location where the diameter will accommodate the endoscope (Figs. 7–6, 7–7); and (4) through a sialolithotomy opening; the endoscope can be inserted through the same opening in the duct where the stone was extracted.
Irrigation during Sialoendoscopy
Irrigation or inflation is crucial in every endoscopy procedure to create an optical cavity. The cavity must be filled with fluid to allow free movement of the instrument, and the area needs to be lavaged to permit good visualization. Isotonic saline is the fluid of choice. An intravenous bag containing isotonic saline is connected to the irrigation port, and the endoscope is moved forward accompanied by a gentle flow of saline. Next, 4 cc of 2% lidocaine is injected through this port, resulting in the anesthesia of the entire ductal system.
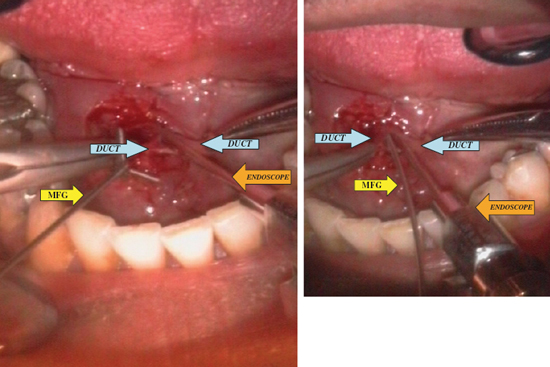
FIGURE 7-7 Demonstration of insertion of the endoscope (diagnostic unit) and surgical instrument (mini grasping forceps, MGF) into the ductal lumen after exploration and exposure of the Wharton’s duct.
Approaches
An intraductal or extraductal approach is possible. The intraductal approach is a purely endoscopic technique. The extraductal approach is an endoscopically assisted technique (Tables 7–1 and 7–2).
Intraductal Sialolithotomy
When a sialolith is encountered, its diameter is estimated using the caliber of the endoscope as a reference, and the method of choice for its removal is selected from four possibilities:
- Removal in one piece by use of grasping forceps, wire baskets, graspers, or balloons (Figs. 7–8, 7–9)
- Crushing the calculus with forceps, then remov ing the fragments using irrigation
- Fragmentation with laser lithotripter
- Combined use of an intracorporeal laser litho tripter or extracorporeal shock wave lithotripter (ESWL), wire basket, and grasping forceps
Table 7-1 Determination of the Appropriate Submandibular Sialoendoscopic Technique
| Stone location and diameter | Technique used |
| Sialolith located up to the middle third of the duct | Sialolithotomy and diagnostic sialoendoscopy |
| Sialolith < 5 mm, located in the hilum area Papillotomy/duct exploration | Grasping forceps |
| Grasper or wire basket or balloon | |
| Lithotripsy (do not use lithotripsy in nonfunctional glands;apply a ductal stretching technique) | |
| Sialolith > 5 mm located in the hilum area | Ductal stretching technique |
| Sialolithotomy | |
| Diagnostic sialoendoscopy and removal of residual sialoliths | |
| Secondary ducts, ductal exploration technique | Grasping forceps |
| Lithotripsy |
Table 7-2 Determination of Appropriate Parotid Sialoendoscopic Technique
| Stone location and diameter | Technique used |
| Sialolith located up to 1 cm from the papilla | Sialolithotomy and diagnostic sialolithotomy |
| Sialolith < 5 mm, located up to the posterior third of the duct Papillotomy/duct exploration | Grasping forceps |
| Grasper or wire basket or balloon | |
| Lithotripsy | |
| Sialolith > 5 mm, located up to the posterior third of the duct | Ductal stretching technique |
| Sialolithotomy | |
| Diagnostic sialoendoscopy and removal of residual sialoliths | |
| Sialolith > 5 mm, located in the middle and up to the posterior third of the duct, and dilation and intraductul stone removal failed | Endoscope-assisted extraoral approach |
| Secondary ducts, ductal exploration technique | Grasping forceps |
| Lithotripsy | |
| Strictures of anterior location | Balloon dilation (two trials) |
| Endoscope-assisted application of grasping forceps |
The primary goal is to remove the calculus in one piece. If this fails, the second option is crushing, and the ultimate resort would be intracorporeal lithotripsy. Occasionally, particularly in cases where lithotripsy has been used or multiple sialoliths were encountered, it has been necessary to perform a second sialoendoscopy to clear the involved gland of all obstructions.
Extraductal Sialolithotomy
The following extraductal approaches are available:
- Intraoral techniques. These techniques can be used for submandibular and parotid stones.
- Extraoral technique. This technique is exclusively for impacted parotid stones.
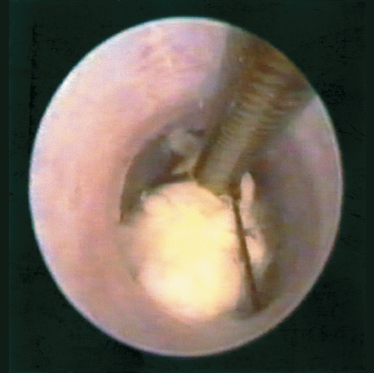
FIGURE 7-8 Endoscopic view of basket retrieval of sialolith from the submandibular hilum.
Intraoral Sialolithotomy
We developed the so-called ductal stretching technique to overcome sialolith removal that we cannot solve with the purely endoscopic techniques or a failed attempt to extract the sialolith by purely endoscopic techniques (Fig. 7–10). In our experience, indications for this technique include:
- Large-size calculi in the submandibular and parotid ducts, measuring more than 5 mm
- Narrowness of the duct, which effectively rules out the option of attempting an intraductal approach
- Failures of the intraductal techniques
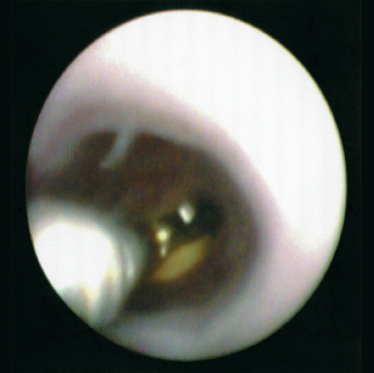
FIGURE 7-9 Endoscopic view of mini grasping forceps retrieval of sialolith from the submandibular hilum.
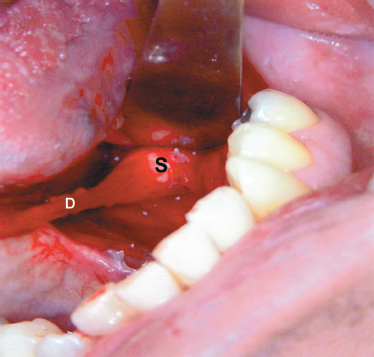
FIGURE 7-10 Ductal stretching technique. A 7 mm stone in the hilum of the submandibular gland with too narrow a duct for pure endoscopic retrieval. D, duct; S, stone.
The ductal stretching technique involves these steps:
- Introducing the endoscope for exact location of the stone lavage and disconnecting the stone from the ductal attachment
- Introducing the lacrimal probe into the duct and making an incision above the duct with a CO2 laser
- Dissecting and isolating the duct from the surrounding tissues up to the first molar (submandibular) or the curvature of the duct above the masseter muscle (parotid)
- In submandibular cases, forwarding the gland toward the mouth with digital pressure from the submandibular region
- Ductal section above the calculus and sialolithotomy
- Endoscopic exploration for removal of additional calculi and lavage
- Temporary polymeric stent insertion for 4 weeks
Extraoral Sialolithotomy
This approach is exclusively reserved for removal of impacted parotid stones.19 The indications for the extraoral approach are:
- Calculus in the posterior third of the Stensen’s duct with too narrow duct anterior to it
- Obstruction of the posterior or middle third of the Stensen’s duct leading to the calculus
- Large-size (> 5 mm) stones in the middle or posterior part of the duct that cannot be dilated for intraductal removal
- Intraparenchymal stones
Identification of Sialolith
The first step is to identify the exact location of the sialolith. There are two main approaches:
- Sialoendoscopic identification
- Ultrasound identification
The endoscopic approach is indicated when there is a possibility of introducing the endoscope (Nahlieli Sialoendoscope, Karl Storz GmbH, Tuttlingen, Germany, Diagnostic Unit 1.3 mm) into the duct. The ultrasound identification is indicated when there is no possibility to penetrate the duct via Stensen’s duct due to ductal obstruction or severe stenosis.
The Calculus Can Be Identified through the Ductal Lumen
Following infiltration of local anesthesia around the orifice of the Stensen’s duct and irrigation of the Stensen’s duct with 2% lidocaine, the diagnostic unit is introduced, and the calculus is identified. The exact location on the outer skin is marked with the aid of the transillumination effect of the sialoendoscope.
The Calculus Cannot Be Identified through the Ductal Lumen
The gland is evaluated with high-resolution ultrasonographic examination (ATL-300, Advanced Technology Laboratory, Bothell, WA with high resolution 5–12 MHz linear probe). The calculus is detected, and its depth, size, and shape are annotated. Skin coordinates are drawn to the exact surgical location of the stone, and a biopsy wire marker is inserted under ultrasound control to locate the stone.
Removal of Sialolith
The suspected area of the stone is infiltrated with local anesthesia. A 1 cm incision according to the facial lines is performed. Sharp and blunt dissection will lead to the stone. If we have a location difficulty problem during the dissection, the ultrasound probe is used.
We reach the capsule or the scar tissue over the duct around the stone. A No. 11 blade is used to open the capsule or the fibrosis, and the stone is exposed and removed with the aid of curettes. A guide is inserted to the cavity of the stone, and a 1.3 mm Nahlieli endoscope is inserted to screen the area and to remove additional particles. A thorough lavage under direct vision is performed. After removal of the stone and the additional particles, a polyethylene stent is inserted into this region directed from the location of the stone intraorally. The stent is fixated with 4.0 silk to the oral buccal mucosa.
In intraparenchymal stones there is no need for stent usage. The capsule is sutured with 4.0 Vicryl suture, skin closure with 6.0 nylon. Pressure dressing is applied for 48 hours. In cases where there is obliteration of the anterior part of the duct and no passage to the oral region is identified, a 1.7 mm vein line is introduced (after removal of the stone) from the location of the stone to the oral mucosa. The needle is removed, and the shaft is incised. Using a 4.0 silk suture, the vein line is fixated to the oral mucosa.
Antibiotic coverage follows the procedure. The procedure is done with the aid of magnification loops × 3.5. Pressure dressing is applied for 48 hours. The length of the procedure is 90 to 120 minutes.
Limitations
The depth of the stone from the outer skin surface should not exceed 6 mm, and screening of the surrounding tissue is mandatory for large blood vessels and for phleboliths. In the presence of deep calculi or close relation to a large-size blood vessel, we recommend exploring this region using a face-lift approach.
Postoperative Management and Care
Following interventional sialoendoscopy, a temporary polymeric stent (sialostent) (Sialotechnology Ltd., Ashkelon, Israel) is introduced into the duct and kept in place for 4 weeks (Figs. 7–11A, B). After placement of the stent, the surgeon continues in submandibular cases to perform a modified anti-kink procedure to correct the unfavorable angle of Wharton’s duct (around the lingual nerve and the mylohyoid muscle). In the author’s opinion, this is one of the main causes of the formation of sialoliths.
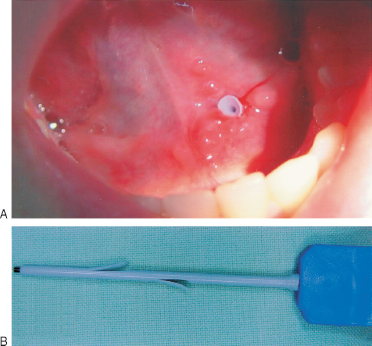
FIGURE 7-11 (A) Sialostent in the Wharton’s duct after endoscopic surgical intervention. (B) Sialostent.
The aim of this procedure is to prevent recurrence of new stones. Ideally, a 4-week period of retention is most desirable. Its purposes are bridging the gap between the healing process of the oral region (the penetration region of the endoscope), which normally occurs very fast, and the restoration of normal function of gland, which normally takes around 2 to 4 weeks, and the prevention of the obstruction of the ductal lumen by postoperative edema. This also allows any calculus fragments to be washed out by the saliva and acts as a stent in an attempt to reduce the possibility of stenosis. Ductal marsupialization that involves suturing the incised ductal margins to the overlying incised mucosal margins can act as an adjunctive measure to provide added safety for maintaining patency of the ductal opening. All patients are treated postoperatively with antibiotics for 7 days.
Outcomes, Success Rate, Failures, and Complications
Over the past 11 years (1993–2004), sialoendoscopy has been performed on 892 salivary glands, with symptoms of obstructive disease. There have been 442 males and 450 females, with ages ranging from 2 to 96 years. There were 598 submandibular glands, 289 parotid glands, and 5 sublingual glands. Eighty-six percent of the glands were diagnosed with obstruction, and 14% with sialadenitis.
All patients underwent preoperative and postoperative screening, including routine radiography, sialography, and ultrasound. Postoperative examination was routinely performed 1 month following the procedure. Some patients were followed as much as 40 months postendoscopy. The majority of procedures were performed under local anesthesia on an outpatient basis. The time for the procedure ranged from 30 to 90 minutes.
The success rate for parotid endoscopic sialolithotomy was 86%, and the success rate for submandibular endoscopic sialolithotomy was 89%. Immediate failures (introduction of the miniature endoscope failed or proved not feasible) accounted for 1.4% of cases. Intraoperative failures (inability to accomplish any of the endoscopic retrieval techniques) were 6%, and late failures 5%. One patient suffered from temporary lingual nerve parasthesia, 1.7% suffered from postoperative infection, 0.4% suffered from postoperative bleeding, 0.9% developed traumatic ranula, and 2.5% suffered from ductal strictures.
Endoscopic Observations and Treatment in Clinical Practice
In clinical practice, several microanatomical and pathophysiological phenomena have been encountered in the course of sialoendoscopic procedures.
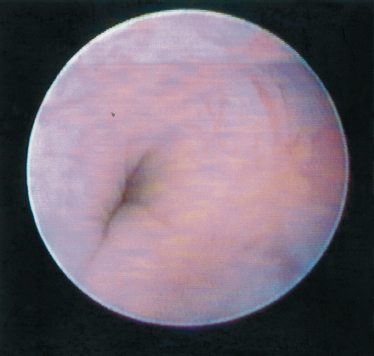
FIGURE 7-12 Sphincter-like system of the Wharton’s duct in the closed position.
Mason and Chisholm, in their book published in 1975, described the presence of smooth muscle strands around the walls of the Wharton’s duct.32 Katz described them in his article in 1991.9 We were able to demonstrate this mechanism and publish in 199711 (Figs. 7–12 and 7–13). Although a search in the literature did not reveal sphincter-like mechanisms in the parotid gland, we were able to observe and document this mechanism in the Stensen’s duct. The difference between the sphincter-like systems in the parotid and submandibular gland is in their location. In the Wharton’s duct, the sphincter-like system begins near the papilla and runs posteriorly. In the Stensen’s duct, it is located posteriorly in the vicinity of the ramification.
During sialoendoscopies we could identify in few cases the sublingual duct opening (Bartholin’s duct) in the Wharton’s duct. This opening was noted in the anterior part of the Wharton’s duct, between 0 and 5 mm posterior to the papilla.
Typical changes in the ductal system during different states of health were noted. In chronic sialadenitis or with long-standing calculi, the lining mucosa of the ductal system had a matted appearance, ecchymosis and a small number of blood vessels. In a healthy gland or in patients with short-term stasis of saliva, there was a shiny appearance of the ductal lining, and proliferation of blood vessels was noted.
Peculiar connections between calculi and the ductal wall were observed in the submandibular and parotid glands. The connections in the Wharton’s duct were found posterior to the bifurcation), the point where the duct divides into the inner and the outer lobes, whereas in the parotid gland, they were posterior to the curvature. No such connections were detected anterior to these regions.
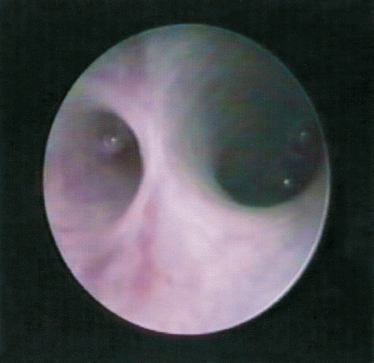
FIGURE 7-13 Open position of the duct; the submandibular gland hilum is observed.
Ductal polyps were noted in 12 glands, 8 in the Stensen’s duct and 4 in the Wharton’s duct. All polyps caused obstructions; four of them in the parotid gland had a history of salivary gland surgery before endoscopy, and three were associated with calculi. All the polyps demonstrated in the sialogram as a filling defect. They were not diagnosed on ultrasound. The polyps were extracted by miniature biopsy forceps or basket.
Intraparenchymal stones located close to the ductal system could be seen with the endoscope, and deeper calculi could not be observed.
We could identify seven foreign bodies in the ductal system, four in the parotid duct and three in the submandibular duct; four of them were hair shafts, and three were parts of a plant most probably (they were washed out during irrigation). Five of them were associated with calculi, and three of them were in children. We observed a formation of sialolith around a hair shaft in two cases.
Due to the more sophisticated equipment and techniques we were able now to better identify, diagnose, and treat the obstructive conditions of ductal strictures and kinks. These malformations were detected in 98 cases, 28 kinks (22 submandibular and 6 parotid) and 70 strictures (26 submandibular and 44 parotid).
We identified an anatomical malformation in the submandibular hilus, a pelvis-like formation (a basin-like structure) instead of a bifurcation or trifurcation. This pelvis formation caused obstructive phenomena and was demonstrated as a widening of the duct in the hilus region in a sialogram.
We revealed an evagination in a 10-year-old child who suffered from two sialoliths. The sialoliths were identified in the Wharton’s duct. During extraction of these calculi from the duct, the formation of an evagination was noted. It obstructed the ductal lumen and was the cause, in our opinion, of the calculi formation. We assume that the intraductal evagination is a form of anatomical malformation.
Instrumentation (Figs. 7–14, 7–15)
We have now progressed to using a semirigid, moderately flexible endoscopic device (Nahlieli Sialoendoscope) specifically designed for salivary gland endoscopy. It is 1 mm in diameter, 10,000 pixels, with two facilities: an exploration unit with an outer sleeve of 1.3 mm, and a surgical unit with a sleeve of 2.3 mm × 1.3 mm, with three channels for introducing a surgical device with a diameter of 1 mm and an irrigation port device.
Another unit is the type 1 endoscope (pistol). The outer sleeve is 2.3 mm, with a 1 mm (200 mm length) telescope, a sleeve for surgical instruments, connection for irrigation pump, and a valve for control. This type of surgical unit is for exploration following sialolithotomy of large-size stones. The surgical instruments that can be useful are instruments from 1 mm or less. The useful tools are grasping forceps, basket, grasper, balloon-like Fogarty or sialoballoon catheter, biopsy forceps, intra-corporeal lithotripter probes, and laser probes. We work under direct vision, so we can use our instruments with meticulous observation. If there is a problem of space and we cannot insert the multichannel endoscope, we can insert the diagnostic unit and the surgical endoscope by its side.
The last option is to work in a semiblind technique, to identify the obstruction with the 1.3 mm diagnostic unit, to remove the 1 mm telescope, and to insert the working instrument through the sleeve. This option is indicated especially in the narrow Stensen’s duct.
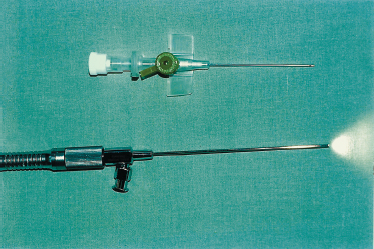
FIGURE 7-14 The 1.3 mm diagnostic unit of the sialoendoscope.
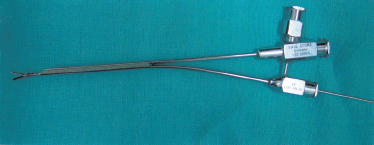
FIGURE 7-15 The 2.3 mm surgical unit with grasping forceps in the surgical sleeve.
A new innovative line of multifunctional instruments (Karl Storz, GmbH, Tuttlingen, Germany) was developed recently with the advantage of a minimal diameter, from 1.1 mm with a channel for irrigation and surgical instruments, to make the sialoendoscopy procedures easier (Fig. 7–16).
Choosing the Appropriate Instrument
A calculus that can be bypassed is usually best handled with the basket. Calculi that cannot be bypassed due to narrowness of space can be handled with a grasping forceps or grasper. In the author’s opinion, the grasping forceps is well controlled, and the stone can be held and maneuvered easily by this instrument. Lithotripter probes are used to fragment the calculus when the other instruments fail. Balloons are good tools, especially for strictures, but also for a soft small calculus. Biopsy forceps are used for ductal polyps.
Sialolithiasis Lithotripsy
Lithotripsy for kidney stones was first reported in 1980. The first report on the use of shock waves to fragment sialoliths was in 1986 by Marmary.33 The problems initially were due to the large lithotripsy machine that had very broad focus. They caused removal of dental fillings and periosteal irritation. There are three external lithotripsy methods depending on the system of generating the shock waves: electrohydraulic, electromagnetic, and piezoelectric. The waves are brought to focus through acoustic lenses. The shock waves pass through a water-filled cushion to the sialolith, where two mechanisms, stress and cavitation, act to fragment the calculus. The soft tissue and the water around the stone do not interfere with the passage of the shock waves. A compressive wave is propagated through the stone, subjecting it to stress. The energy from the sialolith–water contact results in the formation of expansion waves, inducing cavitation bubbles.
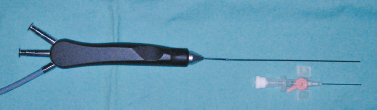
FIGURE 7-16 The 1.1 mm multifunctional sialoendoscope with integrated surgical sleeve and irrigation.
When the bubbles collapse, a jet of water is projected through the bubbles to the surface of the stone. This force is enough to fragment the stone. Development of smaller machines with a more finely focused beam of waves led to a few centers in Europe using it. From 1989 we can find in the literature articles discussing the results of ESWL. The technique delivers 1000 to 5000 shock waves per session. Usually three sessions are needed. The location of the stone is identified and targeted through an inline ultrasound 7.5 MHz probe.
Reviewing the relevant literature34–36 demonstrates a success rate from 16 to 63% for stone-free gland proven by ultrasound screening. Most authors have found elimination of the symptoms is a very significant value of the technique. Iro and colleagues achieved complete stone removal in 50 to 58% of the patients, partial elimination of the stone in 35 to 50%, and alleviation of symptoms in 76 to 100%.34 Escudier et al found 38% stone free and 62% with residual fragments.35 They also found stone size to be a statistically significant indicator of success, ESWL being less effective on stones larger than 7 mm. The morbidity following the lithotripsy procedures is low and includes ductal bleeding, gland swelling, petechial skin hemorrhage, and secondary infection of the affected gland.
Until recently the low success rate and the very expensive equipment were the main obstacles preventing more surgeons from using this technique. The rapid development in miniaturization of the equipment, the reduction in the equipment price, and the combination with other minimally invasive techniques gave this lithotripsy technique a place in our armamentarium in the treatment of sialolithiasis.
In 2004 we (Nahlieli and Hecht-Nakar) developed miniature ESWL (Sialotechnology Ltd., Ashkelon, Israel).37 The diameter of the generator is not bigger than a computer box, and the therapy head was reduced dramatically to fit the dimension of the head and neck region. Ultrasound and endoscopy are used to locate the calculus. The endoscope irrigates and inflates the salivary gland using isotonic saline. Additionally, 2% lidocaine anesthetizes the entire gland and protects the salivary parenchyma, generating more substance to produce the cavitation effect. The success rate of the new lithotripter for complete removal of the stone is 63% (37% total elimination, 26% a need for simple endoscopic intervention). The elimination of the symptoms is striking: Ninety percent of the patients were symptom free following the first treatment. The endoscopic removal of the residual stones after the lithotripsy procedures is easier and less complicated. The shock waves disconnect the stone from the ductal wall and reduce the volume of the stone. In the future, as in urology, ESWL will be in many cases the first line of treatment. This treatment is adjuvant technique for deep stones in the hilum of the submandibular and in the posterior parts of the parotid gland.
Intracorporeal Lithotripsy
In this technique the lithotripsy energy is delivered to the target stone through a fine probe. Several methods used to generate the energy for the lithotripsy procedures include the electrohydraulic technique, pneumoballistic technique, dye pulsed laser, and holmium laser.36,38 The probes are delivered to the location of the stone under the supervision of the endoscope. Electrohydraulic and pneumoballistic techniques are most effective in fragmenting the calculus to small pieces, although the particles are not always small enough for free passage through the ductal lumen. The main disadvantage of these energies is the damage of the shock waves to glandular tissues, especially using the electrohydraulic technique.38 The holmium laser, which is a gold standard technique in urology, also causes severe damage to the surrounding tissues and can easily cause ductal perforation. Another disadvantage of this technology is the high cost of the equipment. A new and promising development in the intracorporeal lithotripsy field is a new generation of lithotripters designed especially for the salivary glands based on erbium: yttrium-aluminumgarnet (YAG) laser technology.39 The advantage of this method is the quality of the fragmentation, to a dust that can easily be washed out from the gland with minimal collateral damage to the surrounding tissues. The fragmentation is done under endoscopic supervision and can be done under local anesthetic as an ambulatory procedure. Other important advantages of this technology are the low cost and the availability of the instruments (the basic laser unit is the same as that for dental use).
Acute Sialadenitis Caused by Sialolith
A complete blockage of the salivary duct by stone can cause a saliva collection, and this can easily be infected. The obstruction can be in the hilum region or by a small fragment of stone that was fractured and moved forward to the narrowest place in the distal portion of the duct. The infection can be moderate with swelling of the gland itself, but severe infections with spread to adjacent anatomical spaces is well documented in the literature.40 The submandibular gland can cause severe infection due to its anatomical location and has the potential to cause airway obstruction (see discussion in Chapter 5).
The bacterial cause of parotid and submandibular infection due to sialolith is mainly Staphylococcus aureus.41 During the acute phase, probing of the involved duct is indicated. Surgical intervention during the acute phase to remove the stone (hot sialolithotomy) is documented in the literature, especially with CO2 laser.28 The author’s personal experience is to remove the stone from the duct only if it is in the anterior part of the duct and the sialolithotomy is a simple procedure without a need for dissection.
Another situation in which the patient can benefit from hot sialolilithotomy is in severe infections from the submandibular gland with a need for incision and drainage. In all the other cases the author’s preference is to “cool” the gland with antibiotic treatment first and to wait with the definitive surgical intervention until the gland is clear from acute infection.
Strictures and Kinks
Following the extensive use of sialoendoscopy, in our department, we encountered new pathologies that cause salivary gland obstruction. From our past and present data, it seems that strictures and kinks are the leading cause of obstruction after sialoliths. Strictures in salivary ducts have been mentioned briefly and anecdotally in the literature, whereas, kinks are a new pathology.17
Buckenham et al42 and Brown et al43 were the pioneers in the use of balloon technique under fluoroscopic guidance. The endoscopic technique simplified the dilatation techniques, as we can use less complicated instruments combined with our ability to work under direct or semidirect vision.
Diagnosis
Strictures were investigated and diagnosed by sialography and sialoendoscopy. Kinks were diagnosed mainly by sialography, and sialoendoscopy was used to rule out other pathologies and to locate the kink. Occasionally sialography, demonstrating a kink, revealed storage of the contrast dye in the area of the kink. During the postevacuation phase of the gland, blockage is noted in the same area. Strictures are quite obvious in sialogram, and they have a typical appearance in sialoendoscopy.
Treatment
Treatment techniques are based on the diagnostic progress made in identifying and locating pathologies when using the sialoendoscope.
Dilatation
The treatment of strictures is based, first of all, on diagnosing and locating the pathologic process. Dilatation can be accomplished by saline hydrostatic pressure balloons or a mini-forceps expansion maneuver. The first attempt to dilate the stricture is undertaken by saline pressure irrigation, through the irrigation port, while introducing and advancing the diagnostic sialoendoscope. If the saline pressure irrigation fails to induce dilatation, the next step is to insert a sialoballoon (Sialotechnology Ltd., Ashkelon, Israel). The sialoballoon has a high-pressure balloon tip of 2 to 4 mm in length, and an outer diameter of 2.5 Fr (< 1 mm). It can be inflated with air up to 4 mm H2O pressure at a maximum of 18 Bar. This pressure is sufficient to dilate most of the strictures.
The balloon can be inserted in either of two ways: under direct vision, if there is enough space to insert the sialoendoscope and the balloon; or in a semi-blind approach, through the telescope channel of the diagnostic sialoendocope in a semiblind technique. Another technique for dilatation of strictures is to expand the involved region with the use of miniature grasping forceps. The technique involves the use of the grasping forceps as a dilator, in that the opened grasping jaws are gently moved in a retrograde fashion along the inner wall of the stricture region. Following the procedure, we inject 100 mg of hydrocortisone solution intraductally.
A sialostent can be inserted to assist in preventing re-creation of strictures; the stent is kept in place for 4 weeks. All patients are treated postoperatively with amoxicillin 1.5 g per day for 7 days.
Anti-kink Procedure
The treatment of kinks, as in strictures, is preceded by a careful diagnostic workup to provide accurate localization of the pathology. In the submandibular kink, a balloon performs contouring of the kink. The next step ahead is the advancement ductoplasty. This is performed by stripping the duct and removing ~5 mm of the anterior part of the duct, pulling it anteriorly, and inserting a sialostent. The anterior edge is sutured to the mucosa and periosteum near the lingual side of the anterior teeth with 4.0 Vicryl suture. For additional support, a 3.0 silk suture is inserted through the oral mucosa lining and the ductal layer and connected to the anterior teeth. The whole maneuver means to extend the angle of the kink. The procedure is completed by injecting 100 mg hydrocortisone into the kink region through the sialoendoscope.
In the event of a parotid kink, the procedure includes balloon contouring, placement of the polyethylene stent, and hydrocortisone injection. All patients are treated postoperatively with oral antibiotics for 7 days. All these procedures are performed under local anesthesia on an outpatient clinic basis.
 Conclusion
Conclusion
Sialoendoscopy is a promising new method for use in diagnosing and treating many inflammatory conditions of the major salivary glands. It is an outpatient procedure, utilizing local anesthesia and without major complications. It appears to be the future solution for the management of perplexing inflammatory salivary gland pathology. As more surgeons become involved with endoscopy, more findings and innovations will be forthcoming, adding to its effectiveness.
REFERENCES
1. Escudier, MP McGurk, M. Symptomatic sialoadenitis and sialolithiasis in the English population: an estimate of the cost of hospital treatment. Br Dent J 1999; 186: 463–466
2. Epker, BN. Obstructive and inflammatory diseases of the major salivary glands. Oral Surg Oral Med Oral Pathol 1972; 33: 2–27
3. Nahlieli, O Eliav, E Hasson, O Zagury, A Baruchin, AM. Pediatric sialolithiasis. Oral Surg Oral Med Oral Pathol 2000; 90: 709–712
4. Rauch, S Gorlin, RJ. Diseases of the salivary glands. In: Gorlin, RJ Goldman, HM, eds. Thoma’s Oral Pathology, 6th ed. St. Louis: Mosby; 1970: 997–1003
5. Lustmann, J Regev, E Melamed, Y. Sialolithiasis: a survey of 245 patients and a review of the literature. Int J Oral Maxillofac Surg 1990; 19: 135–138
6. Frame, JW Smith, AJ. Large calculi of the submandibular salivary glands. Int J Oral Maxillofac Surg 1986; 15: 769–771
7. Hardy, KJ. Submandibular calculus disease at the Royal Melbourne Hospital, 1954–1963. Med J Aust 1966; 16: 670–671
8. Konigsberger, R Feyh, J Goetz, A Schilling, V Kastenbauer, E. Endoscopic controlled laser lithotripsy in the treatment of sialolithiasis. Laryngorhinootologie 1990; 69: 322–323
9. Katz, P. Endoscopie des glands salivires [Endoscopy of the salivary glands]. Ann Radiol (Paris) 1991; 34: 110–113
10. Nahlieli, O Neder, A Baruchin, AM. Salivary gland endoscopy: a new technique for diagnosis and treatment of sialolithiasis. J Oral Maxillofac Surg 1994; 52: 1240–1242
11. Nahlieli, O Baruchin, AM. Sialoendoscopy: three years’ experience as a diagnostic and treatment modality. J Oral Maxillofac Surg 1997; 55: 912–918
12. Nahlieli, O Baruchin, AM. Endoscopic technique for the diagnosis and treatment of obstructive salivary gland diseases. J Oral Maxillofac Surg 1999; 57: 1394–1401
13. Hasson, O Nahlieli, O. [Endoscopy of the salivary glands (sialendoscopy): new method of sialolithiasis treatment]. Revista APCD 1998; 52: 277–279
14. Nahlieli, O Baruchin, AM. Endoscopic technique for the diagnosis and treatment of obstructive salivary gland diseases. J Oral Maxillofac Surg 1999; 57: 1394–1401
15. Nahlieli, O Baruchin, AM. Long term experience with endoscopic diagnosis and treatment of salivary gland inflammatory diseases. Laryngoscope 2000; 110: 988–994
16. Marchal, F Becker, M Dulguerov, P Lehmann, W. Interventional sialoendoscopy. Laryngoscope 2000; 110: 318–320
17. Nahlieli, O Shacham, R Yoffe, B Eliav, E. Diagnosis and treatment of stricture and kinks in salivary gland ducts. J Oral Maxillofac Surg 2001; 59: 484–490
18. Nahlieli, O. Development and application of microsalivary gland endoscopy. In: MacGurk, M Renehan, A, eds. Controversies in the Management of Salivary Gland Disease. Oxford: Oxford University Press; 2001: 274–283
19. Nahlieli, O London, D Zagury, A Eliav, E. Combined approach to impacted parotid stones. J Oral Maxillofac Surg 2002; 60: 1418–1423
20. Nahlieli, O Shacham, R Bar, T Eliav, E. Endoscopic mechanical retrieval of sialolithiasis. Oral Surg Oral Med Oral Pathol 2003; 95: 396–402
21. Blair, GS. Hydrostatic sialography. Oral Surg Oral Med Oral Pathol 1973; 36: 116–130
22. Yune, HY Klatte, EC. Current status of sialography. Am J Roentgenol Radium Ther Nucl Med 1972; 115: 420–428
23. Koischwitz, D Gritzmann, N. Ultrasound of the neck. Radiol Clin North Am 2000; 38: 1029–1045
24. Yoshimura, Y. Sonographic examination of sialolithiasis. J Oral Maxillofac Surg 1989; 47: 907–912
25. Angelelli, G Flavia, G Macarini, L, et al. Echography in the study of sialolithiasis. Radiol Med (Torino) 1990; 79: 220–223
26. Brown, JE Escudier, MP Waites, EJ Drage, NA Ng, SY. Intra-oral ultrasound imaging of a submandibular duct calculus. Dentomaxillofac Radiol 1997; 26: 252–255
27. Klutmann, S Bohuslavizki, KH Kroger, S, et al. Quantitative salivary gland scintigraphy. J Nucl Med Technol 1999; 27 (1): 20–26
28. Azaz, B Regev, E Casap, N Chisin, R. Sialolithectomy done with CO2 laser: clinical and scintigraphic results. J Oral Maxillofac Surg 1996; 54: 685–688
29. Milton, CM Thomas, BM Bickerton, RC. Morbidity study of submandibular gland excision. Ann R Coll Surg Engl 1986; 68: 148–150
30. Mra, Z Komisar, A Blaugrund, SM. Functional facial nerve weakness after surgery for benign parotid tumours: a multivariate statistical analysis. Head Neck 1993; 15: 147–152
31. Owen, ER Banerjee, AK Kissin, M Kark, AE. Complications of parotid surgery: the need for selectivity. Br J Surg 1989; 76: 1034–1035
32. Mason, DK Chisholm, DM. Salivary Glands in Health and Disease. London: WB Saunders; 1975
33. Marmary, Y. A novel and non-invasive method for the removal of salivary gland stones. Int J Oral Maxillofac Surg 1986; 15: 585–587
34. Iro, H Benzel, W Zenk, J Fodra, C Heinritz, HH. Minimally invasive treatment of sialolithiasis using extracorporeal shock waves. HNO 1993; 41: 311–316
35. Escudier, M Katz, P Capacccio, P. Extracorporeal shock wave lithotripsy for salivary calculi: results and future role in the management of salivary calculi. In: McGurk, M Renehan, A, eds. Controversies in the Management of Salivary Gland Disease. Oxford: Oxford University Press; 2001: 257–270
36. Brown, JE. Minimally invasive techniques for the treatment of benign salivary gland obstruction. Cardiovasc Intervent Radiol. 2002; 25: 345–351
37. Nahlieli, O Hecht-Nakar, L. Extracorporeal shockwave lithotripsy as an adjuvant therapy for sialoendoscopy. Laryngoscope 2005; In press
38. Arzoz, E. Salivary gland endoscopy and intracorporeal lithotripsy. In: McGurk, M Renehan, A, eds. Controversies in the Management of Salivary Gland Disease. Oxford: Oxford University Press; 2001: 271–274
39. Nahlieli, O: Selective sialolithiasis lithotripsy. J Oral Maxillofac Surg 2005;In press
40. Lerner, DN Troost, T. Submandibular sialadenitis presenting as Ludwig’s angina. Ear Nose Throat J 1991; 70: 807–809
41. Berry, LR. Sialadenitis and sialolithiasis diagnosis and treatment. Oral Maxillofac Surg Clin North Am 1995; 7: 479–503
42. Buckenham, TM Page, JE Jeddy, T. Technical report: interventional sialography–balloon dilatation of the Stensen’s duct stricture using digital subtraction sialography. Clin Radiol 1992; 45: 34–35
43. Brown, AL Shepherd, D Buckenham, TM. Per oral balloon sialoplasty: results in treatment of salivary duct stenosis. Cardiovasc Intervent Radiol 1997; 20: 337–342
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


