Fig. 6.1
Experimental strategies for mechanistic and developmental therapeutic studies of head and neck cancer stem cells. The head and neck squamous cell carcinoma (HNSCC) from a patient is digested immediately after surgery and sorted for cancer stem cell markers. Following sorting, the cells are transplanted subcutaneously in immunodeficient mice to generate patient-derived xenograft (PDX) tumors. Sorted cells can also be plated in low-attachment culture conditions to generate orospheres and maintain the cancer stem cell phenotype. Both the orospheres and the PDX tumors can be passaged for studies of self-renewal properties of the cancer stem cells
Using these experiments, cancer stem cells were first isolated in HNSCC by Prince and collaborators [37]. In this groundbreaking work, Prince and colleagues sorted varying dilutions of lineage-negative CD44+ or CD44− primary HNSCC cells and implanted them subcutaneously in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice or Rag2/cytokine receptor common γ-chain double knockout (Rag2γDKO) mice. They found that 20 of the 31 transplantations of the CD44+ cells yielded tumors where as only 1 of the 40 CD44− transplantations formed tumors suggesting that the CD44+ were more tumorigenic than the CD44− cells. As few as 5000 CD44+ primary tumor cells were able to generate tumors. In contrast, a minimum of 500,000 CD44− primary cells was necessary for tumor growth. CD44+ generated tumors were serially passaged showing that these cells are capable of self-renewal. When the tumors were digested and flow analyzed, both CD44+ and CD44− cells were seen suggesting that the CD44+ cells are capable of differentiation. Primary CD44+ cells showed a significant upregulation of Bmi-1 expression. Sections taken from primary human tumors showed significant co-staining of CD44 with the squamous epithelial stem cells markers cytokeratin 5/14 further suggesting that these cells did indeed display a stem cell-like phenotype [37]. Collectively, this work unveiled the presence and function of a subpopulation of HNSCC tumor cells with uniquely high tumorigenic potential, self-renewal, and multipotency.
In another important study, Clay and colleagues showed that aldehyde dehydrogenase (ALDH) could also be used as a marker to enrich cancer stem cells in HNSCC. In this study, Clay and colleagues [5] found that FACS-sorted ALDHhigh primary HNSCC cells were significantly more tumorigenic compared to the ALDHlow primary HNSCC cells when transplanted into NOD/SCID mice. Primary HNSCC cells sorted for high ALDH activity were able to form tumors in 24 of the 45 transplantations, while the cells sorted for low ALDH activity formed only 3 tumors out of 37 transplantations. Importantly, as few as 50–100 ALDHhigh cells were able to form tumors. Notably, ALDHhigh cells were able to generate tumors that showed a similar histology when compared to the original unsorted tumors and were able to replicate the original tumor heterogeneity for ALDH activity suggesting that ALDHhigh cells were capable of both self-renewal and multipotency [5].
As both CD44 and ALDH activities have been described to enrich for cancer stem cells in HNSCC, Krishnamurthy and colleagues combined the two markers to determine if they could further enrich for this cell population. In these studies, it was found that ALDH+ CD44+ cells were capable of forming colonies in soft agar more efficiently than ALDH-CD44+ and ALDH-CD44− cells, suggesting that this population was predominately stemlike compared to the other subpopulations. These results led them to perform further in vivo experiments where they FACS sorted 1000 ALDH+ CD44+ cells and 10,000 ALDH-CD44− cells and co-transplanted them subcutaneously in immunodeficient mice together with human endothelial cells in biodegradable scaffolds [23]. They found that the ALDH+ CD44+ cells were able to generate tumors in 13 of the 15 total transplants, while the ALDH-CD44-Lin cells were only able to form tumors in only 2 out of the 15 transplants. To investigate whether these cells were capable of self-renewal, they digested the tumors into single cell suspensions and serially transplanted into immunodeficient mice. ALDH+ CD44+ cells were able to generate secondary tumors, whereas the ALDH-CD44− cells were unable to form secondary tumors again suggesting that these cells were capable of self-renewal. The fraction of ALDH+ CD44+ cells remained low in both the primary and secondary tumors suggesting once again that these cells were multipotent [23].
In addition to ALDH and CD44, HNSCC cancer stem cells can also be isolated using a DNA binding dye called Hoechst 33342. When taken up by the cell, this dye binds the DNA and can be seen in FACS analysis. However, cells that upregulate drug-resistant cell transporter proteins, such as ABCG2, exclude the dye and can be sorted out by FACS. These cells are termed side population (SP) cells. In a study by Song and colleagues [44], they found that SP cells were able to form significantly more spheres in clonogenic soft agar assays when compared to non-SP cells. When SP and non-SP cells were transplanted in vivo, the SP cells were able to form tumors using as few as 100 cells, while 10,000 non-SP cells were required to initiate tumor growth [44]. Tabor and colleagues [45] also found this side population of cells in HNSCC cell lines. When they sorted the side population and re-plated the sorted cells into new tissue culture flasks, the SP cells were able to differentiate and generate non-SP cells suggesting that SP cells were multipotent. In addition to multipotency, they also noted that SP cells showed an increased ability to form spheres under nonadherent conditions suggesting that SP cells were also capable of self-renewal. When SP cells were transplanted into mice, they were able to generate tumors using 5000 SP cells. In contrast, no tumors were observed using 5000 non-SP cells suggesting that SP cells were uniquely tumorigenic [45].
Several studies have also proposed the use of other cancer stem cell markers in HNSCC. One study suggested that cells containing low levels of reactive oxygen species may also be uniquely tumorigenic [3]. Other markers that have been suggested include CD10 and CD271 [12, 31]. In their studies, Fukusumi and colleagues found that CD10+ HNSCC cells were significantly more sphere forming in vitro and tumorigenic in vivo. Using HNSCC cell line Detroit562, they were able to generate tumors in all of the six CD10+ transplants, whereas only two of the six CD10 transplants developed tumors. However, this difference in tumorigenicity was not seen in the FaDu HNSCC cell line, suggesting that CD10 might be a cell line-specific CSC marker. Murillo-Sauca and colleagues sorted CD271+ cells alone or in combination with CD44+ cells and transplanted these cells subcutaneously into Rag−/−γc−/− mice. They found that when they transplanted 10,000 CD271+ and 10,000 CD271 cells, they were able to generate tumors in three of the five CD271+ implants, whereas no tumors were generated in the CD271 cells. When CD271+ CD44+ cells were transplanted, they were able to generate tumors using as few as 100 cells. The CD44-CD271 cells were only able to generate tumors when 1000 cells were transplanted.
The search for the ideal marker(s) for head and neck cancer stem cells is far from complete. Ideally, a specific marker or marker combination would select for highly tumorigenic cancer cells, and absence of these markers would identify cancer cells that do not have tumorigenic potential. Such specificity has not been achieved yet. Further, it will be critical to understand if CSC markers only have the ability to identify stem cells, or if they might play a functional role in the making of a cancer stem cell. This is perhaps relatively clear when one thinks about SP cells, where the function of a key drug-resistant cell transporter protein is upregulated. This might explain, at least in part, the observation that cancer stem cells are highly resistant to chemotherapeutic drugs such as cisplatin [34]. On the other hand, a possible functional role for other CSC markers (e.g., CD10, CD44) appears less clear. Nevertheless, this is an area of intense research that should yield more conclusive results in the upcoming years.
6.3 Signaling Pathways in HNSCC Cancer Stem Cells
Much research has been done to characterize the pathways that regulate the cancer stem cell phenotype in HNSCC. For example, a recent study showed that the Wnt/β-catenin signaling axis is critical for the maintenance of the stem cell phenotype [26]. Wnt signaling plays an important role in normal stem cell function during embryonic development by modulating differentiation, migration, and proliferation [6]. In their investigations, Lee and colleagues found that both cytoplasmic and nuclear β-catenin were seen in a small subpopulation of HNSCC cells. This staining overlapped with ALDH1 and CD44 staining suggesting that the β-catenin activity is active and primarily restricted to cancer stem cells. Indeed, when β-catenin was overexpressed in HNSCC cell lines, they saw an increased sphere formation as well as an increase in expression of the stem cell markers Oct-4, Sox2, and CD44. Importantly, overexpression of β-catenin increased in expression of ABC transporters as well as significantly increased chemoresistance to cisplatin treatment. In contrast, when β-catenin was knocked down, they saw a significant reduction in sphere growth and a decreased expression of Oct-4, Sox2, CD44, and the ABC transporters. Importantly, knockdown of β-catenin significantly reduced the tumorigenic potential of HNSCC cells in vivo. Interestingly, overexpression of Oct-4 restored the tumorigenic potential in vivo upon knockdown of β-catenin, suggesting that β-catenin regulation of the cancer stem cell phenotype occurs in part through Oct-4 [26]. In agreement with this study, work by Song and colleagues found that SP cells have significantly higher Wnt/β-catenin signaling than non-SP cells. In their studies, they used a TOPFLASH luciferase reporter with wild-type β-catenin binding sites and found increased activity of β-catenin-dependent transcription. They also found that DKK1 and AXIN2, two critical Wnt/β-catenin target genes, were upregulated using PCR analysis [44]. Collectively, this work provides strong support for the functional role of the Wnt/β-catenin signaling pathway in the pathobiology of head and neck CSCs.
Several other studies have elucidated the importance of Oct-4 in HNSCC cancer stem cell function. Ventelä and colleagues found that cells expressing Oct-4 had a less differentiated phenotype and were more resistant to chemotherapy. Indeed, patients with high Oct-4 expression do have lower survival rates than patients who do not [48]. In agreement with this study, Koo and colleagues found that overexpression of Oct-4 in several HNSCC cells lines significantly increased cell proliferation and sphere formation. Notably, cells overexpressing Oct-4 were more resistant to cisplatin treatment. These cells had greater expression of the stem cell markers Sox2 and Nanog as well as the ABC transporter protein. Oct-4 overexpression also increased the invasive potential of these cells and elevated the levels of slug, an important epithelial to mesenchymal transition (EMT) transcriptional factor. Notably, Oct-4high cells showed increased tumorigenic potential in vivo, when compared to Oct-4low cells [21].
Another important signaling pathway in cancer stem cell biology is mediated by interleukin (IL)-6, an important inflammatory cytokine. This pathway was first characterized in breast cancer stem cells by a study from Sansone and colleagues [41]. In their investigations they found that antibody blockage of the IL-6 binding to the IL-6 receptor (IL-6R) significantly decreased secondary mammosphere formation in low-attachment conditions, suggesting that this ligand-to-receptor interaction is important in the self-renewal of breast cancer cells. Conversely, when IL-6 was added to primary sphere cultures, these investigators observed an increase in secondary mammosphere production. Interestingly, the MCF-7 cell line-derived mammospheres showed increased expression of IL–6 mRNA when compared to the normal attachment MCF-7 cells suggesting that the breast cancer stem cells significantly upregulate IL-6 when compared to the bulk tumor cells. Further experiments suggested that IL-6 binding activates the Notch-3 pathway, an important signaling axis in the regulation of stem cell function [41]. The Poliak laboratory further elucidated the role that IL-6 plays in the function of breast cancer stem cells [30]. In their study they found that IL-6 is important in many stem cell self-renewal pathways. In particular, IL-6 reduction led to a reduction in phosphorylated STAT3, which has been shown to be important for maintaining stemness in murine embryonic stem cells.
IL-6 was also found to be important in HNSCC specifically in predicting the recurrence and survival rates among HNSCC patients. In an epidemiological study by Duffy and colleagues [9], they compared pretreatment IL-6 serum levels and correlated these data to the posttreatment clinical outcomes of the patient. They found that patients with high IL-6 pretreatment serum levels had lower survival and a higher rate of disease recurrence, suggesting that IL-6 may be an important biomarker for HNSCC aggressiveness and risk for recurrence [9]. IL-6 signaling was also found to be important in HNSCC cancer stem cells. Krishnamurthy and colleagues found that IL-6R was significantly upregulated in the ALDHhighCD44high cancer stem cell population, when compared with controls [24]. Importantly, tumors in IL-6 wild-type mice grew significantly faster than tumors grown in IL-6 knockout mice. Notably, HNSCC xenograft tumors generated in the IL-6 wild-type mice showed higher fraction of ALDHhighCD44high cells, suggesting that stromal IL-6 plays an important role in the maintenance and self-renewal of head and neck cancer stem cells in vivo. This effect was mediated in part by the activation of STAT3 signaling. Interestingly, studies from Islam and colleagues [16] suggested that inhibition of RhoC expression could downregulate the STAT3 pathway, indicating that this protein may also be involved in IL-6-driven maintenance of the CSC phenotype. In these studies, knockdown of RhoC suppressed sphere formation, decreased the percentage of ALDHhigh cells, and decreased the level of phospho-STAT3. Addition of IL-6 to the RhoC knockdown cells restored levels of phospho-STAT3 in HNSCC cell lines. They concluded that RhoC activated downstream pathways (possibly NF-κB) that induced transcription of IL-6, which then activated downstream STAT3 signaling and the maintenance of the CSC phenotype [16].
Bmi-1, a member of the polycomb repressive 1 complex, is another protein that plays an important role in normal stem cell function [35]. It was also found to be important for the maintenance of cancer stem cells in HNSCC [34], particularly upon treatment with a chemotherapeutic drug (cisplatin). Treatment of HNSCC with cisplatin significantly increased the population of ALDHhighCD44high cells in a dose-dependent manner and increased their ability to form spheres in vitro. Interestingly, this increase in the cancer stem cell fraction correlated with an increase in Bmi-1 expression. A study by Giudice and colleagues [14] further elucidated the role of Bmi-1 in HNSCC. They showed that HNSCC cells are typically hypoacetylated. Chemical inhibition of histone deacetylase significantly decreased sphere formation and the fraction of ALDHhighCD44high CSC. Paradoxically, chromatin hyperacetylation induced Bmi-1 expression and epithelial to mesenchymal transition (EMT), suggesting that the regulation of Bmi-1 through histone acetylation in HNSCC tumor cells may be important for the transition from a more stemlike state to a more motile and invasive state [14].
6.4 Cancer Stem Cell Niche
Stem cell niche is the specific microenvironment that allows stem cells to retain their stemness and give rise to progenitor cells. Like normal stem cells, CSCs are known to reside in niches (Fig. 6.2). Stem cell niches consist of endothelial cells, fibroblasts, immune cells, signaling molecules secreted from different types of cells, and extracellular matrix [22]. Cancer stem cell niches allow them to maintain the population and provide protective environment against cancer therapies [11, 15]. Here, we will discuss the role of fibroblasts and endothelial cells within head and neck cancer stem cell niches.
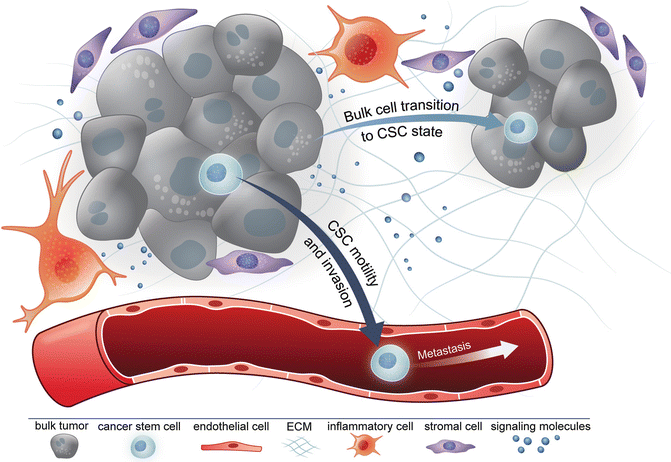

Fig. 6.2
Cancer stem cell niche. The cancer stem cell niche is a protective environment including multiple cell types where cancer stem cells reside. The interaction between cancer cells and the stromal cells allows the cancer stem cell population to retain stemness. Such interactions might also enhance invasiveness of cancer stem cells enabling them to enter into the bloodstream and disseminate through the process of metastases. It has been hypothesized that environmental cues might enable the dedifferentiation of more differentiated tumor cells back to a cancer stem cell state
6.4.1 Cancer-Associated Fibroblasts
Stromal cells are important components of most tumor microenvironments and play a key role in the pathobiology of cancer. Factors secreted by tumor cells result in cancer-associated fibroblasts (CAF) that are phenotypically distinct from normal fibroblasts (reviewed in [20]). Cancer-associated fibroblasts, along with other cells within the cancer stem cell niche, activate stemness-related pathways. Vermeulen and colleagues found that stromal myofibroblasts activated canonical Wnt pathway to regulate the stemness of cancer cells [49]. In lung cancer, CAF-activated IGF-II/IGFR signaling pathway enhanced the stemness of cancer cells [4]. Stromal contribution in head and neck cancer has also recently been proposed to play a role in tumor cell invasion [29]. Stromal cell-derived factor (SDF-1) secreted by fibroblasts induces migration of head and neck CSCs to supportive niches. SDF-1 is also involved in podia formation, which is needed for cell interaction with the microenvironment [10]. SDF-1 is a strong chemoattractant that plays an important role in tumor metastasis [13, 36, 46]. Collectively, these findings suggest that cancer stem cells acquire enhanced stemness and motility through CAF-induced molecular signaling. Such interactions might ultimately contribute to tumor progression and dissemination.
6.4.2 Perivascular Niche
Work in brain cancers suggested the existence of a cancer stem cell niche, perhaps inspired by the existing knowledge that normal neural stem cells reside near the blood vessels. Endothelial cells secrete factors that allow neural stem cells to maintain the ability to self-renew [17, 38]. As has been observed in normal neural stem cells, brain cancer stem cells in glioblastoma multiforme are in close proximity with endothelial cells [2]. When patient-derived brain cancer stem cells were injected with vascular endothelial cells to immunodeficient mice, the CSCs were able to maintain their stemness and tumorigenicity [2].
In HNSCC, cancer stem cells reside in perivascular niche [23]. Close proximity between cancer stem cells and blood vessels enables active crosstalk between the two cell types. Factors secreted by endothelial cells potentiate the survival and self-renewal of CSC [23]. Specifically, endothelial cell-secreted IL-6 is important in maintaining the tumor-initiating ability of CSC as well as in population maintenance within the tumor [24]. In addition to IL-6, endothelial cells also secrete high levels of epidermal growth factor (EGF). EGF enhances orosphere formation and increases the motility of HNSCC in vitro [53]. In addition, specific silencing of EGF expression in tumor-associated endothelial cells decreased the fraction of cancer stem cells and the tumorigenic potential in preclinical models of HNSCC. Collectively, these findings suggest that factors secreted by the perivascular niche contribute to the maintenance of the CSC population and the acquisition of a more aggressive phenotype by HNSCC cells.
6.4.3 Epithelial-Mesenchymal Transition and Cancer Stem Cells
Epithelial-mesenchymal transition (EMT) happens when cell of epithelial origin acquires phenotypes resembling mesenchymal cells. Cells that have undergone EMT present enhanced migratory and invasive ability as well as resistance to apoptosis [19]. EMT is involved in cancer initiation and progression in many different cancer types (reviewed in [7, 47]). Several studies linked EMT with the conversion of noncancer stem cells into cancer stem cells. Mani and colleagues [28] were the first to report that induction of EMT results in increased proportion of cancer stem cells, sphere-forming ability, and tumorigenicity in preclinical models of breast cancer. Head and neck CSC are also reported to have mesenchymal morphology [25
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


