Fig. 15.1
Exposed, necrotic left mandible (BRONJ) in a patient with multiple myeloma and a history of zolendronic acid therapy
A clinical staging system developed by Ruggiero [21] and adopted by the American Association of Oral and Maxillofacial Surgeons (AAOMS) in 2006 [22] has served to categorize patients with BRONJ, direct rational treatment guidelines, and collect data to assess the prognosis and treatment outcome in patients who have used either intravenous or oral bisphosphonates. Since the publication of these treatment guidelines, reports of non-specific signs and symptoms such as pain, abscess formation, altered sensory function, or osteosclerosis have emerged in patients with a history of bisphosphonate use but no clinical evidence of necrosis. In an effort to determine whether or not these findings represent a precursor for clinical disease, the recently updated AAOMS position paper has included these patients in a new stage 0 category (Table 15.1) [23]. The proportion of patients with stage 0 disease who will progress to overt BRONJ remains to be determined and represents an important area for future investigations.
Table 15.1
BRONJ staging
|
At risk
|
No apparent exposed/necrotic bone in patients who have been treated with either oral or intravenous bisphosphonates
|
|
Stage 0
|
Non-specific clinical findings and symptoms such as jaw pain or osteosclerosis but no clinical evidence of exposed bone
|
|
Stage 1
|
Exposed/necrotic bone in patients who are asymptomatic and have no evidence of infection
|
|
Stage 2
|
Exposed/necrotic bone associated with infection as evidenced by pain and erythema in the region of the exposed bone with or without purulent drainage
|
|
Stage 3
|
Exposed/necrotic bone in patients with pain, infection, and one or more of the following: pathologic fracture, extra-oral fistula, or osteolysis extending to the inferior border or sinus floor
|
Multiple risk factors, including drug-related issues (potency and duration of exposure), local risk factors (dentoalveolar surgery), local anatomy, concomitant oral and systemic disease, demographic factors, and genetic factors, have all been considered for this complication, but only three risk factors have remained constant throughout most clinical studies. In the majority of BRONJ cases reported to date, recent dentoalveolar trauma was the most prevalent and consistent risk factor [24–26]. Patients with a history of inflammatory dental disease, e.g., periodontal and dental abscesses, are at a seven fold higher risk for developing BRONJ [27]. The duration of bisphosphonate therapy also appears strongly related to the likelihood of developing necrosis, with longer treatment regimens associated with a greater risk of disease development [26, 27]. In addition, the more potent intravenous bisphosphonates that are administered on a monthly schedule, such as zolendronic acid and pamidronate, are significantly more problematic than other preparations.
Efforts to establish risk assessment by measuring fluctuations in bone-turnover markers remain controversial [28–32]. The rationale for this approach is based on the knowledge that markers for bone remodeling will increase within months following withdrawal of oral bisphosphonate medications, suggesting a gradual normalization of osteoclastic function and bone remodeling [33, 34]. However, these markers are a reflection of total bone-turnover throughout the entire skeleton and are not specific to the maxilla or mandible, where it is suspected that the bone-turnover rate may be more severely depressed from prolonged bisphosphonate exposure. From a more practical perspective, using bone-turnover markers to estimate the level of bone-turnover suppression is only meaningful when the values are compared to baseline, pre-treatment levels, and these are rarely obtained in clinical practice. In addition, using the levels of bone-resorption markers to assess the BRONJ risk can be misleading in the small cohort of patients that develop osteoporosis despite normal baseline levels of these markers.
The radiographic features of BRONJ remain relatively non-specific. In fact, plain-film radiography does not typically demonstrate any abnormality in the early stages of the disease due to the limited degree of decalcification that is present. However, findings on plain-film imaging, such as localized or diffuse osteosclerosis or a thickening of the lamina dura (components of stage 0), may be predictors for future sites of exposed, necrotic bone. The findings on computed tomography (CT) are also non-specific but this modality is significantly more sensitive to changes in bone mineralization and therefore is more likely to demonstrate areas of focal sclerosis, thickened lamina dura, early sequestrum formation, and the presence of reactive periosteal bone (Fig. 15.2). CT images have also proved to be more accurate in delineating the extent of disease, which is very helpful for surgical treatment planning [35, 36]. The utility of nuclear bone scanning in patients at risk of BRONJ has received growing attention following reports of increased tracer uptake in regions of the jaws that subsequently developed necrosis [37, 38]. While nuclear imaging has limited value in patients with existing disease, it appears to have some level of potential benefit as a predictive tool in those patients with pre-clinical disease (stage 0) and therefore requires continued evaluation.
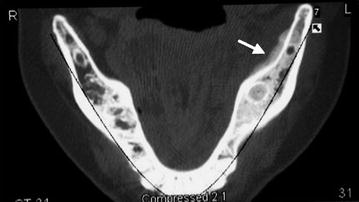

Fig. 15.2
Axial computed tomography view of the mandible in a patient with a history of breast cancer and established BRONJ. The arrow depicts an area of reactive periosteal bone formation on the lingual aspect of the left mandible
15.5 Treatment
The management of patients with BRONJ remains very challenging since surgical and medical interventions may not eradicate the disease. The goal of treatment for patients at risk of developing BRONJ or who have active disease is to preserve the quality of life by controlling pain, managing infection, and preventing the development of new areas of necrosis. This has to be balanced with the oncologic management of the patient with osteolytic metastases and with the risk of pathologic fracture in the osteoporotic patient.
The treatment approach for patients with stage 1 disease has remained primarily non-surgical since these patients do not have evidence of infection nor are they symptomatic. In most stage 1 patients, the exposed bone will eventually mature into a defined sequestrum that can be easily removed. Since infection and pain are typical for patients with stage 2 disease, these patients will benefit from local to systemic antibiotic therapy. They will likewise develop sequestrum, which in most cases can be managed with local debridement. In patients with stage 3 disease, the extensive nature of the necrosis and infection usually dictate early surgical treatment (segmental resection or marginal resection) for the control of infection and pain. Several institutions recently reported that early surgical treatment, regardless of disease stage, is associated with a good level of cure and disease control. This suggests that surgical treatment may soon play a larger role in managing this complication [39–41].
Alternative surgical and non-surgical approaches to treatment have recently emerged and may be of value. Hyperbaric oxygen therapy (HBO), as an adjunct to non-surgical or surgical treatment, is currently being evaluated at several institutions. The preliminary results from a pilot study suggest some improvement in wound healing and pain scores but the routine use of HBO as an effective adjunct or primary treatment modality requires further evidenced-based review [42, 43]. The use of platelet-rich plasma as an adjunct to local resection and primary closure was reported in a total of five cases at two separate institutions [44, 45]. In all instances, there was complete wound healing and resolution of pain. However, the small number of reported cases and the lack of controls mandate that further study of platelet-rich plasma is needed before it can be utilized therapeutically in BRONJ. In a single case report, the administration of systemic low-dose parathyroid hormone (PTH), an anabolic bone hormone, was successful in resolving an area of necrosis when other modalities of treatment had failed [46]. In a recent prospective, placebo-controlled study of 40 patients, low-dose systemic PTH in conjunction with vitamin D and oral calcium was associated with greater resolution of periodontal bone defects and accelerated intraoral osseous healing [47]. Although PTH is contraindicated in patients with osteolytic bone metastases, these promising findings may have real applicability for BRONJ patients in the non-cancer setting.
For those few patients who require surgical resection, the reconstruction of BRONJ-related defects has been challenging. Although there have been reports of immediate reconstruction with vacularized bone grafts, most surgeons are hesitant to proceed with such a procedure given the uncertain viability of the remaining native bone [39]. In those instances, the mandible is stabilized with a reconstruction plate and soft-tissue flaps [48]. The use of bone morphogenetic protein within a sponge carrier has been described for immediate reconstruction of discontinuity defects in BRONJ patients and might represent a viable alternative to conventional grafting techniques in this patient population [49].
If risk factors are truly predictors of disease, then modification of such factors should translate into a change (reduction) in disease severity or occurrence. In patients at risk of developing BRONJ, adherence to risk-reduction protocols has been reported to decrease the incidence of this complication [50]. Implementation of a detailed dental assessment and the avoidance of dentoalveolar surgery during treatment with zolendronic acid resulted in a five fold reduction of osteonecrosis [51]. In those instances in which BRONJ developed, the application of stage-specific treatment protocols resulted in a manageable level of disease and good symptom control in a large majority of patients [52]. Studies are underway at several institutions to determine whether dose-reduction schedules for zolendronic acid in the setting of cancer treatment will lower the incidence of BRONJ while retaining the oncologic effectiveness of the drug.
15.6 What We Don’t Know
As we examine our present understanding of BRONJ as a therapy-associated complication and project our concerns into the future, there are many questions that remain to be answered. The recent emergence of anti-RANKL antibodies as a valid bone-targeted therapy for patients with cancer and osteoporosis has created a new set of potential challenges since these drugs also appear to be associated with the development of ONJ. While the reversible nature of this novel anti-resorptive therapy may prove to be uniquely beneficial in the management of affected patients, those potential benefits have yet to be studied or confirmed.
Accurate predictors of the disease also remain elusive. While certain types of nuclear imaging may prove to have some predictive value for those patients at risk, it is still not clear who should receive such a costly and invasive test or when it should be performed. As yearly zolendronic acid therapy (Reclast) becomes increasingly accepted, assessment of the risk of BRONJ takes on added urgency. Based on current studies, the risk of developing this condition was found to be very low through three years of bisphosphonate treatment [53, 54]. However, these data need to be compared with those from conventional oral bisphosphonate therapy in which a significant risk of developing necrosis only appears after a drug-exposure history longer than 3 years. More importantly, the risk of developing BRONJ in the setting of monthly intravenous zolendronic acid therapy for cancer has been well described. However, the degree and timing (if any) of risk-reduction following the cessation of therapy remain poorly understood. In addition, a better understanding of the exposure thresholds for intravenous (and oral) therapy is required so that patients and clinicians can be more accurately informed of the potential risks of these bone-targeted treatments.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


