Liquid/powder
Glass/ceramic
Zn O
Pre-polymerized resin powderb
Phosphoric acid
Silicate cement
Zinc phosphate cement
Acrylic acid
Glass-ionomer cement
Zinc polycarboxylate cement
Eugenol
Zinc oxideeugenol cement
Resin
Resin-composite cement
Resin and phosphoric acid
Glass phosphonate cement
Resin and acrylic acid
Resin-modified glass ionomer
MMA monomera
Unfilled Resin cement
Yet, most contemporary cements are based on glass filler. For glass-ionomerbased cements the glass should be acid soluble to enable ion discharge to react with the acrylic acid. The liquid component of most cement is responsible for adhesive properties. Generally, cements that contain only a resin need an adhesion promoter to create adhesion to a substrate, whereas, for instance, glass-ionomerbased cements can create a true chemical bond to the dental hard tissues.
Another way to classify dental luting cements is by dividing them into conventional and adhesive cements. The conventional cements or the non-adhesive cements are cements that do not have the potentiality to bond to tooth structure. The “adhesive” luting cements can be divided into two main groups, that of cements that have the intrinsic property to create chemical bonding to the tooth tissues through ionic exchange, and that of cements that can obtain this bond principally by micromechanical interlocking with the conditioned tooth structure. The first group consists mainly of glass-ionomer cements and resin-modified glass-ionomer cement. The second group consists mainly of resin-based cements, which include unfilled adhesive resin cements and composite resin cements. The composite resin cements generally are composed of polyfunctional dimethacrylate-based monomers, such as Bis-GMA and/or urethane dimethacrylate, and inorganic filler of fine glass or ceramic and silica. In other words, their composition is analogous to that of resin-composite restoratives but with a lower filler loading. Actually, in the latter group the adhesive properties are determined by the type of the combined adhesive system and not primarily by the choice of the cement per se. Concern is often raised regarding the risk of pre-curing the bonding agent prior to the insertion of the luting composite on the fit of the indirect restorations. To solve this problem, several ways are possible, firstly, by taking the impression of the prepared tooth after the application and light curing of a bonding agent, and secondly, by utilizing dual-curing bonding agents that do not necessitate separate light curing before cementation. Finally, this problem can be solved by using resin-composite cements that do not need separate adhesion promoters as they utilize self-etching primers and inherent adhesive promoter monomers in the resin component of the lute itself, e.g., Panavia F cement and Bistite II DC cement.
The unfilled resin cements, on the other hand, performs as both the adhesive system and the luting media; hence, for these cements a diffusion promoter monomer is often added to the liquid of the cement, which enables the infiltration of the conditioned dentin. This diffusion promoter is a bifunctional molecule containing hydrophilic and hydrophobic groups. Although the strength of unfilled resin cements is inferior to that of composite resin cements, they have the advantage of being bonded directly to the tooth structure without needing a separate bonding-agent step that might compromise the restoration’s fit. In addition, unfilled adhesive resin cements based on MMA monomer are more elastic than those made of polyfunctional, cross-linked polymer chains. The most widespread example of unfilled adhesive resin cement based on MMA monomers is the 4-META/MMA-TBB resin cement (Super-Bond C&B, Sun Medical or C&B Metabond, Parkell). Fig 7.1 gives an overview of the classification of luting cements.
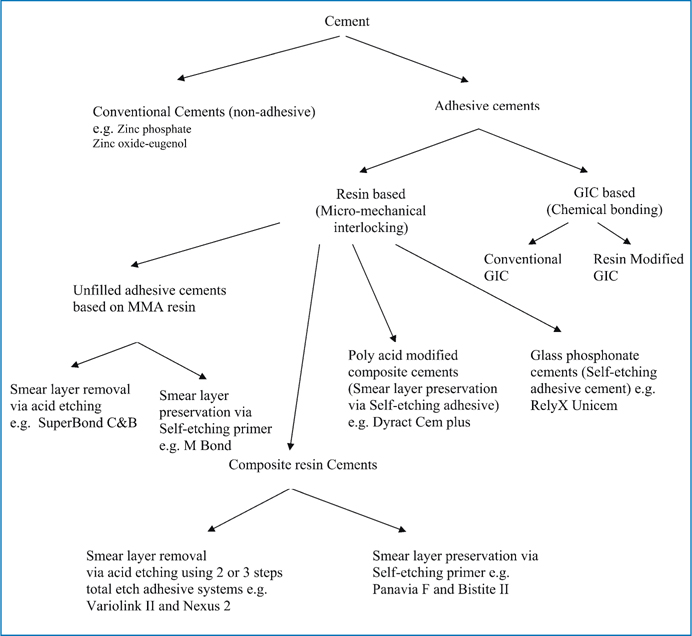
Fig. 7.1
Classification of Luting cements based on adhesion mechanism to tooth structure
Strength and Solubility
Strength and solubility of the cement is an important parameter for the longevity of the cemented restoration. The conventional zinc ortho-phosphate cements were prone to dissolve in saliva. For that reason a small cement film thickness at the margin is attempted for these cements. Despite the fact that resin-based cements do loose some of their contents when exposed to water, they are hardly soluble; therefore, a very tight fit of restoration cemented with the latter resins is not mandatory. When the cement margins are in the occlusal area, the cement may be prone to occlusal wear. For that reason highly filled cements may be required. As the filler content and size influences the viscosity of the unset cement negatively, special composite cements are developed the viscosity of which can be temporarily lowered by applying ultrasound energy on the restoration to be placed utilizing its thixotropic properties.
Stability and Stiffness
All cements in dentistry do undergo dimensional changes during and after setting. During setting the closer distance between reacted molecules does cause a setting shrinkage, whereas the exposure of the set cement to the oral environment may result in water sorption and swelling. As a reliable bond of the cement to the substrates is attempted, both dimensional changes may lead to stress development at the cement substrate interface. Generally, the setting shrinkage stresses may place the adhesion at risk, whereas the hygroscopic expansion may affect the integrity of all-ceramic restorations. Also, the modulus of elasticity of the cement is of importance for mainly the brittle all-ceramic restorations. Ceramic restorations need a stiff cement, such as resin-composite cement, for providing good support. In contrast, the low modulus of elasticity of the unfilled MMA-based adhesive resin cement offers resiliency and flexibility to the bond with higher resistance to occlusal impact stresses, which is more relevant for splinting loose teeth or cementing adhesive bridges.
Bonding to Tooth Structure
Buonocore in 1955 showed that etching enamel with phosphoric acid leads to loss of superficial enamel with preferential dissolution of the underlying enamel leading to the creation of microporosities [3]. By applying unfilled bonding resins to the etched enamel, the resin can be drawn by capillary attraction into the microporosities to form a resin-enamel interlocked composite layer. This bond is still one of the most reliable; however, the development of self-etching primers and adhesives that require the omitting of the separate etching step may result in insufficient resin-enamel bond due to the weak acidity of some mild self-etching systems. This concern is more relevant when there is a large surface enamel-adhesive interface area such as facial laminate veneers or adhesive bonded-bridge restorations. To tackle this drawback, application of the self-etching primer during a sufficiently long time of at least 15 s and actively applying it through rubbing the enamel surface with repeated applications of fresh material is suggested [4]. Alternatively, a separate step of acid-etching procedure can be accomplished prior to the application of the self-etching primer.
In contrast to bonding to enamel, bonding to dentin is more technically sensitive and is affected by several variables. After preparation of dentin, nearly always a smear layer, consisting of inorganic material and embedded in organic matrix, develops. The layer tenaciously adheres to the prepared dentin surface. For that reason the smear layer and the underlying dentin has to be conditioned to enable resin to penetrate or remove the smear layer and to bond to the underlying dentin. Resin-based adhesive systems utilize different methods to condition dentin. The first method attempts to remove the smear layer completely via acid etching and rinsing. The second approach aims at preservation of the smear layer; however, in both approaches the adhesion is based on micromechanical interlocking by creating a so-called hybrid layer. Among the contemporary adhesive systems are cements that employ self-etching primers that are based on the latter approach (Fig 7.2). The use of these adhesive systems is an outcome of efforts made to simplify the bonding procedure and to improve the bonding quality by reducing the number of required steps in the bonding procedure. When applied to smear layers, these resin systems demineralize the smear layer and incorporate it into the applied resin, which slightly penetrates into the underlying dentin, thereby creating a hybrid layer in which the undissolved collagen fibers of the dentin are incorporated and which contain the remnants of the original smear layer [5]; thus, the separate steps of using an acid and primer are combined in one procedure. Moreover, a number of contemporary adhesive resin-based cements that utilize selfetching primers do not make use of the separate resin-bonding-agent phase that might affect the fitting of the indirect restorations. More recently, self-etching adhesive resin-based cement was developed (RelyX Unicem, 3 M ESPE). The organic matrix of this cement consists of multifunctional phosphoric acid-modified (meth)-acrylates. This adhesive cement does not employ pretreatment of the tooth surfaces as it depends on the inherent acidity of the resin matrix to condition the tooth surface. The Unicem cement can be categorized as glass phosphonate cement as it is related to silicate cements due to the incorporation of basic inorganic filler within the matrix which participates in the cement reactions together with the acidic groups of the monomer.
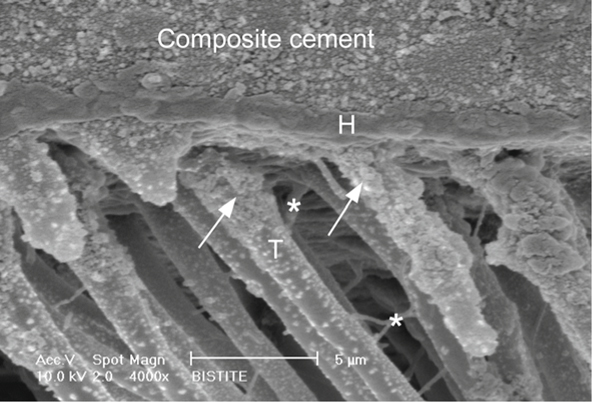
Fig. 7.2
Scanning electron microscopy photomicrograph of resin-dentin interface produced by Bistite-II cement which utilizes self-etching primer. The luting composite is attached to the dentin surface by an acid-resistant hybrid layer (H) of 0.6-1 µm. The hybrid layer is extended into the tubule wall forming tubule-wall hybridization (arrow) at the top of the resin tags (T). Lateral branches (asterisk) are formed from the main resin tags representing the high infiltration potentiality of that cement.
Nevertheless, resin cements that utilize self-etching approach generally suffer from the weak acidity of their acidic resin in comparison with phosphoric acid; therefore, it is widely believed that the bonding performance of these systems could be affected by the quantity and quality of the smear layer [6]. Clinicians routinely use diamond burs for extracoronal preparations followed by impression taking and provisional restoration using temporary cements. It was found that bond strength to dentin prepared with diamond burs and conditioned with selfetching primers was lower than that prepared with fine-fissure steel burs and conditioned with the same system. Alternatively, the reduction in bond strength was not observed when the smear layer created by different burs was removed with adhesives that utilize phosphoric acid conditioning [7]. For these reasons, it might be useful to finish the preparation steel burs.
Bonding to Ceramic
Bonding of adhesive resin cement to ceramic can be achieved through micromechanical and/or chemical bonding mechanisms. There are several methods to condition ceramic surfaces to enhance bonding to resin-luting cements, although the effects of different surface treatments on bonding are strongly dependent on the type and the microstructure of the ceramic surface to which to bond [8 – 10].
Surface Preparation
Mechanical bonding to ceramic surfaces can be enhanced by preparing the surface by grinding, abrasion with a diamond rotary instrument, airborne-particle abrasion with aluminum oxide, and etching using different types of acids. Hydrofluoric acid (HF) is commonly used to etch porcelain for indirect restorations [11, 12]. As alternatives, to avoid the hazardous HF, acidulated phosphate fluoride [8] or phosphoric acid (H3PO4) were also used to condition the ceramic surfaces; however, their effectiveness on the enhancement of the bond strength is still doubtful [13]. Phosphoric acid is used in industry to etch glass at high temperature. At room temperature the action of H3PO4 is limited to clean the ceramic surface without producing an apparent etching pattern (Fig 7.3), and therefore this treatment does not contribute to the resin ceramic bond strength [14].
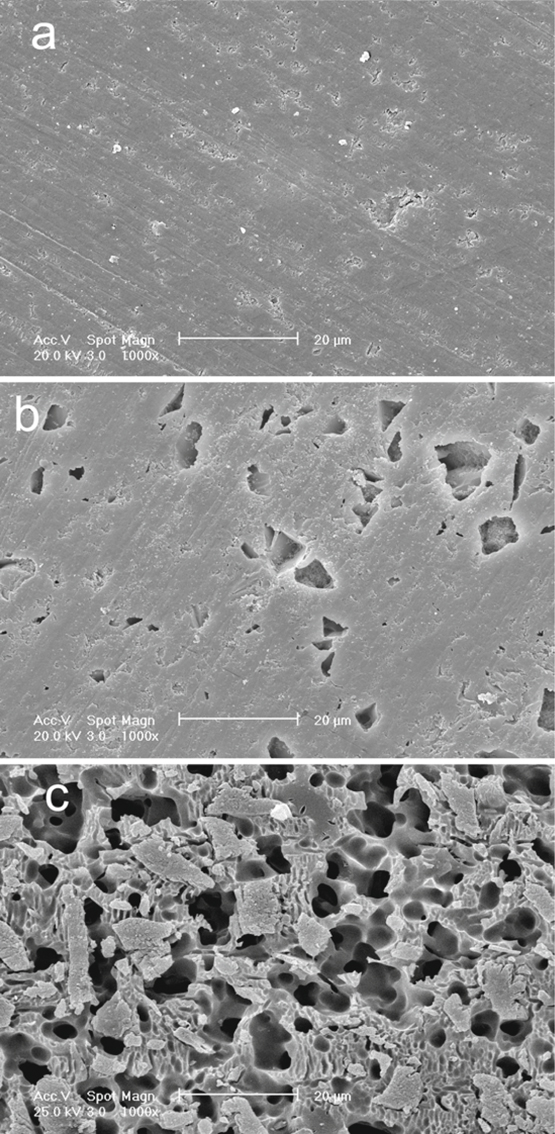
Fig. 7.3
a-c. Scanning electron micrographs of the Vita blocs Mark-II ceramic surfaces. a Specimen after 600-grit SiC wet grinding.b Specimen treated with 37 % H3PO4. Note that there are no apparent etching patterns; the surface shows only exposed surface porosities and defects caused by the cleaning action of the acid. c Specimen treated with 8 % HF. The etching pattern with numerous undercuts for micromechanical retention is clearly seen
The capability of HF to alter the ceramic surface depends on the ceramic microstructure and composition. Ceramic that contains a glass phase (leucite, silica-based feldspath, or glass ceramics) can be etched with HF, whereas all-ceramic restorations made of aluminous cores cannot be etched sufficiently. The HF creates a surface pattern for micromechanical attachment by preferential dissolution of the glass phase from the ceramic matrix which increases the surface area and enhances the micromechanical retention of the resin cement [15]. The micro-undercuts formed on the ceramic surface by HF etching allow the penetration of both the resin and filler components of the luting composite cement to form particle-reinforced resin tags that contribute to strong resin-ceramic bond [16]. Due to this ability of the filler to penetrate the micro-undercuts without being filtered, the resin ceramic bond strength can substantially increase by treating the etched ceramic with filled bonding agent [17]. On the other hand, over-etching with high HF concentrations or extended etching times may lead to a reduced bond strength [18], or HF can be so aggressive to the surface of some ceramic materials that it declines its mechanical properties, which would in turn affect the resin-ceramic bond strength [9, 19]. Consequently, one should take into account the type of ceramic being used before HF etching.
Alumina blasting is used to remove refractory investment material during the laboratory procedures of the fired ceramic restorations when the hot-press technology is used. For these cases the ceramic surface is always gently roughened. It was found that the bond strength of porcelain laminate veneer was greater when etched than when lightly sandblasted [12]. Conversely, excessive sandblasting to improve the bond can induce chipping and adversely affect the fit of all-ceramic restorations without significantly improving bond strength [20, 21].
Chemical Bonding
For effective and durable resin ceramic bond, not only micromechanical bonding, but also a chemical bonding, should be attempted. The most common and effective way to achieve a chemical resin-ceramic bond is through using silane coupling agents. Silane coupling agents are bifunctional molecules that improve the wettability of the ceramic surface and form a covalent bond with both the ceramic and the resin cement [22]. Silane agents commonly consist of g-methacryloxypropyl trimethoxysilane (γ-MPTS). The reaction between methoxy silane groups of γ-MPTS and OH groups of the porcelain surface that formed siloxane bonds can be initiated and accelerated by using acid catalysis [23]. Presently, contemporary ceramic primers utilize separate acidic catalyst liquids, such as monomer of 10-methacryloyloxydecyl dihydrogen phosphate (MDP), or carboxylic compound. When the acidic catalyst is mixed with the silane coupler component, the methoxyl groups hydrolyze to initiate stable siloxane bond (Si-O-Si) with the porcelain surface (Fig 7.4) [24].
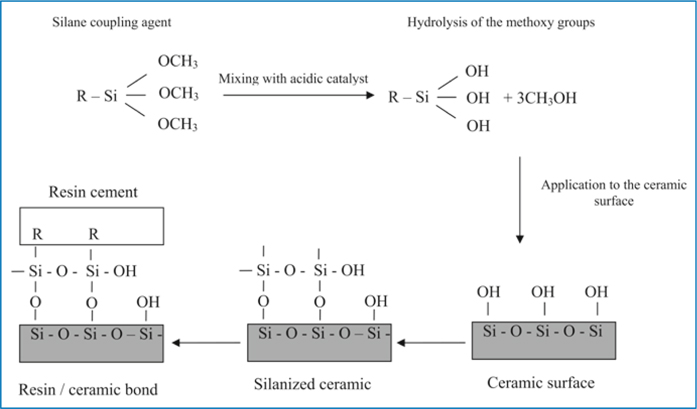
Fig. 7.4
Silane treatment procedure
Accordingly, ceramic primers can be classified as follows [25]:
1.
Unhydrolyzed single-liquid silane primer
2.
Prehydrolyzed silane primer that is also single liquid
3.
Two- or three-liquid primer with separate silane coupler and acid activator
The single-liquid prehydrolyzed silane primer has shown a better bonding performance than the unhydrolyzed form; however, the stability of the prehydrolyzed silane primer appears to be insufficient and its shelf life is limited compared with the multicomponent liquid primers [25].
Improvement in ceramic resin bond strength can also be accomplished through heat treatment of the silanized porcelain. It is believed that during heating (100°C for 60 s) water and other contaminates, such as alcohol or acetic acid, are eliminated from the silane-treated surface, which drives the silane/silica surface condensation reaction towards completion and promotes silane silica bond formation [21].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


