Blood plasma
Publisher Summary
This chapter describes the physiological and chemical characteristics of blood plasma. The blood plasma constitutes the intravascular portion of the extracellular fluid of the body. It amounts to some 3.5 liters, and represents, therefore, about one quarter of the total volume of extracellular fluid and about 5% of total body weight. The main cation in the plasma, as in extracellular fluids generally, is sodium. The effective plasma osmotic activity exerted across the walls of the capillaries is much less than 700 kPa as the capillary walls are permeable to small molecules and to water. Plasma differs from the other extracellular fluids in having an appreciable content of proteins. The protein content of plasma amounts to about 75 g/1 of plasma. The distribution of fluid throughout the body depends largely upon the osmotic pressure differences between fluid compartments. Plasma proteins may bind substances and facilitate their transport in the blood. Chronologically, the first stage in blood clotting is the production of the thrombokinase complex. Normal human serum contains an α2 globulin that can exhibit activated C’1 esterase. Deficiency of this inhibitor may be a factor in the hypersensitivity reaction of angioneurotic oedema.
The blood plasma constitutes the intravascular portion of the extracellular fluid of the body. It amounts to some 3.5 litres, and represents, therefore, about one quarter of the total volume of extracellular fluid, and about 5% of total body weight. Plasma is separated by centrifuging whole blood: it is the fluid layer above the packed cells (Fig. 3.1). It normally constitutes around 55% of the blood volume. Plasma differs from serum, the fluid obtained after centrifuging blood that has been allowed to clot, in its greater content of fibrinogen and some of the clotting factors.
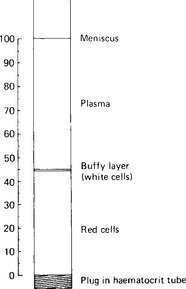
Figure 3.1 A haematocrit tube after centrifugation. The proportions of cells (denser than plasma, and therefore at the bottom), and plasma (at the top), can be read off as a percentage of the blood volume. The buffy layer includes the white cells and the platelets.
Composition-inorganic
Although the concentrations of inorganic ions in plasma may vary in different parts of the body, they are in general little different from those in extracellular fluid in the tissues (Table 1.4). The blood serves principally as a transport medium, carrying materials around the body, and acts, therefore, as an equilibrating system throughout the body. The values for plasma concentrations given in this chapter are reasonably representative values: they might be different, for example, in blood leaving the absorptive areas of the gut, or in blood entering or leaving the kidneys. It should be remembered here and throughout the book that most values given as normal are figures which are somewhere towards the middle of the normal range but are rounded out or chosen as being fairly easy to remember, rather than precise as in a physical science. It would often be preferable to give normal ranges, but these are less convenient to hold in one’ mind, and even then may vary among different populations.
The main cation in the plasma, as in extracellular fluids generally, is sodium. The usually accepted value for its concentration is 152 mmol/l of plasma water, or 140 mmol/l of plasma. This distinction is made because the proteins of plasma contribute to its volume, so that 1 litre of plasma contains only about 930 ml of water. Potassium concentrations in extracellular fluids are low; the values usually given for plasma are around 5 mmol/l of plasma water, and around 4.7 mmol/l of plasma. The other major cations of plasma are calcium, at 2.45 mmol/l of plasma, and magnesium, at 1.5 mmol/l of plasma. The pH of plasma is around 7.40, the hydrogen ions being completely balanced by an excess of hydroxyl ions. Variation in pH occurs as carbon dioxide concentration varies: arterial blood in the lungs has a pH of about 7.40, and mixed venous blood returning to the heart, carrying more carbon dioxide in solution, has a pH of about 7.36. The inorganic ions of plasma are important in maintaining fluid balance in the body since they contribute to the total plasma osmotic pressure exerted across the effectively semi-permeable walls of the cells. Some of the plasma ions help to maintain the concentrations of ions in cells and fluids at optimum levels for the correct functioning of specific body systems – calcium ion concentrations, for example, affect the activity of nerves and muscles.
The total osmotic activity of plasma
The effective plasma osmotic activity exerted across the walls of the capillaries is much less than 700 kPa since the capillary walls are permeable to small molecules as well as to water (see p. 22, and the section below on functions of plasma proteins).
The plasma proteins
Concentrations and classification
The protein content of plasma amounts to about 75 g/l of plasma. The plasma proteins contribute little to the total osmotic activity of the blood because their large molecular size means that relatively few molecules, or solute particles, are present in that total – in comparison, for example, with sodium ions whose molecular weight is about 3000 times smaller but whose concentration is only 20 times smaller (see Fig. 3.2).
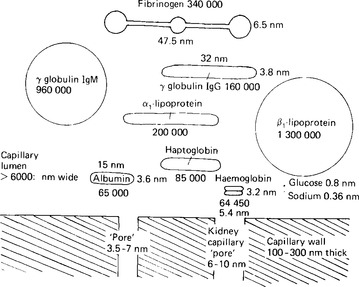
Figure 3.2 The relative sizes of the plasma constituents. The relation of these sizes to that of the apparent pores in the capillary walls is shown by the size of pore indicated at the bottom of the diagram.
The original classification of proteins into albumin and globulins was based on fractionation of serum by precipitation, at first by ammonium sulphate, later by various alcohol mixtures. Since serum was used, this classification excluded the fibrinogen present in plasma. The use of increasingly sophisticated electrophoretic techniques, which separate molecules on a basis of charge and size, has led to the separation and identification of more and more fractions. This in turn has allowed identification of proteins by function as well as by physical properties. As a result, the globulins have been subdivided into α1 and α2, β and γ globulins, and pre-albumin fractions have been described.
The globulins
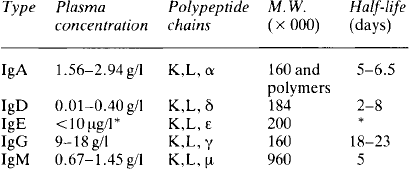
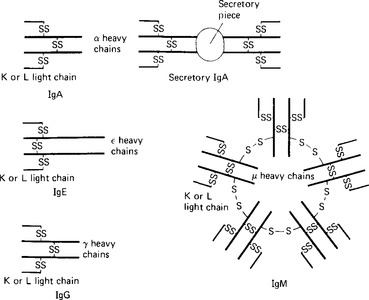
The γ globulin fraction is the second largest of the main protein fractions of plasma; the plasma concentration in a normal healthy adult subject is 6-12 g/l. γ globulins are large molecules, with molecular weights between 160000 and 960000 daltons. Five main classes of γ, or immunoglobulins are recognised, all based on a structure consisting of two pairs of ‘heavy’ and ‘light’ polypeptide chains (Table 3.1). The ‘light’ K and L chains are each built up from two subunits of 110 aminoacids and each of the ‘heavy’ α, γ, δ, ε and μ chains consist of four of these subunits. The proteins differ in the types of chain they contain and in the structure formed by these chains (Fig. 3.3).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses