
15

Atlas of Salivary Gland Surgery
Surgical resection is the most commonly employed treatment modality for tumors of the salivary glands. Safe and effective surgery of the salivary glands is dependent on a detailed knowledge of anatomical relationships in the head and neck and requires precise surgical technique. Surgical anatomy is described in Chapter 1, but pertinent details will be highlighted where appropriate in the following description of surgical technique. The primary goal of surgical therapy obviously is treatment of the disease, but the greatest emphasis must be placed on preservation of function. Examples of potential functional consequences of surgical resection include facial nerve dysfunction after surgery for parotid gland tumors and dysfunction of the lower cranial nerves after surgery for tumors of the parapharyngeal space. These nerve deficits can markedly impact the patient’s quality of life, and therefore a great deal of consideration must be given before deliberate sacrifice of a functioning nerve. As a general rule, elective resection of a functioning nerve can be justified only if there is direct infiltration by a malignant tumor, and if the involved nerve is the only anatomical site precluding total resection of the tumor. Rehabilitation of patients after nerve section can be difficult, and this issue is addressed in Chapter 16. The reader is referred to Chapters 9 and 10 for a detailed discussion on the decision-making process in the treatment of salivary gland tumors. The focus of this chapter will be to describe in some detail the most commonly indicated surgical procedures for the management of parotid, submandibular, parapharyngeal, and minor salivary gland tumors. The technical details of ancillary surgical procedures such as neck dissection and its various modifications, sentinel node biopsy for intraparotid nodes, temporal bone resection, and combined craniofacial resection are beyond the scope of this chapter and have been well described elsewhere.1
 Surgery of the Parotid Gland
Surgery of the Parotid Gland
The decision for surgical excision of a tumor of the parotid gland is mainly undertaken based on the extent of the tumor and the patient’s medical fitness for the procedure. Advanced, surgically unresectable malignant tumors are treated with external beam radiation or combined chemoradiation therapy. All other tumors are amenable to resection using one of the procedures listed in Table 15-1 The aim of surgical resection is to achieve complete resection of the tumor, and the amount of normal parotid gland removed in the process is largely dependent on the location of the tumor within the gland and its relationship to the branches of the facial nerve. Although the nature of the tumor may influence the extent of resection in some instances, this decision is generally based on the clinical and/or radiologic findings.
The role of diagnostic imaging (Chapter 2) in the evaluation of tumors of the parotid gland has been debated extensively. Preoperative ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) rarely alters the management of small tumors of the superficial parotid lobe.2 Assessment of large parotid tumors, tumors with decreased mobility, tumors of the deep lobe, patients with preoperative facial nerve dysfunction (rare with benign tumors), and recurrent tumors is enhanced by imaging.
Table 15-1 The Nomenclature of Surgical Operations for the Parotid Gland
| Superficial parotidectomy |
| Limited (partial) superficial parotidectomy |
| Extracapsular dissection |
| Deep lobe parotidectomy |
| Accessory parotid resection |
| Radical parotidectomy |
| Extended radical parotidectomy |
Evaluation of the third dimension of the tumor and its relationship with the plane of the facial nerve is helpful in planning surgery and counseling patients in selected situations. It is also useful in certain situations to determine the relationship of tumor or residual parotid tissue to the plane of the facial nerve. Radiologic demonstration of normal-appearing parotid tissue at the deep margin of a residual or recurrent superficial lobe tumor following previous surgery may provide some assurance that the tissue planes around the nerve are intact. Imaging can also be helpful to evaluate the status of the regional lymph nodes if a malignant tumor is suspected.
Fine-needle aspiration cytology (FNAC) is a simple technique that can provide diagnostic information in selected circumstances (Fig. 15–1). Martin and Ellis at Memorial Sloan-Kettering Hospital introduced FNAC in the United States for cancer and allied diseases in 1930,3 with subsequent interest in salivary gland needle biopsy reported from the Karolinska Institute in Stockholm.4 It became popular in the United States in the mid 1970s. The first goal of FNAC is to determine the presence or absence of neoplasm, secondly to determine if the neoplasm is benign or malignant, and lastly to define the exact type of tumor present. CT-guided fine-needle aspiration can be helpful for nonpalpable lesions. Seeding of the needle tract with an appropriate-sized needle is extraordinarily unlikely.
Many surgeons do not perform fine-needle aspiration in the evaluation of a salivary gland mass, arguing effectively that all salivary gland masses require resection to exclude neoplasm and that the extent of surgery can be defined based on clinical judgment. Benign as well as low-grade, low-stage tumors of the superficial lobe of the parotid are adequately treated by a limited resection. Higher grade, high-stage tumors need more extensive resection. These attributes of the tumor can almost always be reliably estimated on clinical and/or radiologic examination.
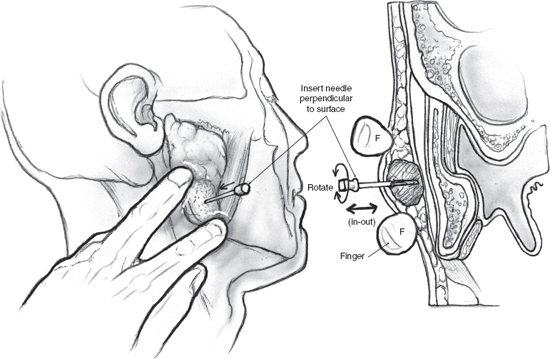
FIGURE 15-1 The technique of fine-needle aspiration cytology (FNAC) for a tumor of the parotid gland.
However, fine-needle aspiration can often distinguish between lesions of salivary gland and nonsalivary origin. The sensitivity to the presence of a neoplasm and the specificity to the absence of a neoplasm are as high as 98% with fine-needle aspiration.5 If of salivary gland origin, aspiration can usually define benign versus malignant and can frequently make a specific diagnosis.6 Distinguishing between benign and malignant tumors approaches 90% in most series, with overall accuracy as high as 97%.5 Determining the exact diagnosis of a salivary gland mass can be as high as 70% in the hands of a very experienced salivary gland cytopathologist. Distinction between salivary tumors and lesions of nonsalivary origin, and between benign and malignant neoplasms, is useful in planning therapy.7 A cytological diagnosis of malignancy is certainly helpful in preoperative counseling, and the surgeon may be able to alert the patient that operative findings dictate sacrifice of the facial nerve. The proliferation of highly trained cytopathologists and advancements in immunohistochemistry have made fine-needle aspiration an important consideration, but the breadth of histological subtypes in salivary gland tumors continues to make specific preoperative cytological diagnosis a formidable goal.
The key to successful FNAC is immediate evaluation of the specimen for adequacy. Immediate interpretation of the cytological specimen by a well-trained cytopathologist can lead to focused studies such as flow cytometry to exclude lymphoma. The experience of the cytopathologist, the person performing the FNAC, and the preparation of the slides are all factors in the accuracy of the procedure. The immediate evaluation of FNAC can be an obstacle for the non-hospital-based clinician, who would require scheduling the procedure at the hospital. For medical-legal reasons, many cytopathologists do not perform fine-needle aspiration, signifying that in some settings the patient’s time, the cytopathologist’s time, and the clinician’s time would have to be scheduled. FNAC for salivary gland lesions must be decided by the surgeon on a case-by-case basis.
The superficial lobe contains the bulk of the volume of the parotid gland, so that most tumors arise from this part of the gland. Superficial parotidectomy or partial superficial parotidectomy is therefore the most common surgical resection required in the treatment of parotid tumors. Superficial parotidectomy is described in detail because the technique of this operation should be used as the basis for any lesser resections that might be possible depending on the location of the tumor within the gland. Partial superficial parotidectomy is safe in selected situations, and proponents of its routine use point to the fact that a substantial proportion of tumors resected by a complete superficial parotidectomy will have exposure of the tumor capsule where the tumor has been dissected off the facial nerve or its branches.8,9 Local recurrence rates after superficial parotidectomy are dramatically lower with partial or complete superficial parotidectomy compared with those after the tumor has been enucleated without formal dissection of the facial nerve. There is a menu of procedures such as extracapsular dissection (ECD) and partial superficial parotidectomy (PSP) that are available to the surgeon between the extremes of enucleation and superficial parotidectomy (see Chapter 9). Enucleation of a parotid tumor, even if it is obviously benign, results in unacceptably high rates of local recurrence and should not be practiced. There is considerable literature on the relevance of ECD in the management of parotid tumors, but this author does not personally prefer the procedure.
Another area of controversy in surgical technique involves the role of monitoring of the facial nerve. Successful dissection and preservation of the facial nerve and its branches in a previously unviolated surgical field depend largely on the surgeon’s technical expertise and familiarity with surgical anatomy. The surgeon can use the nerve monitor (or disposable nerve stimulator) to demonstrate intact function of the dissected nerves at the conclusion of parotidectomy. Facial nerve monitoring for primary cases of mobile parotid tumors of the superficial lobe, less than 4 cm in size, has been reported not to reduce the risks of permanent or transient facial nerve dysfunction.10 Other retrospective series have shown a lower rate of facial nerve dysfunction with facial nerve monitoring.11,12 A threshold of intraoperative facial nerve stimulation has not been identified that would predict postoperative facial nerve function.13 Facial nerve monitoring should be encouraged in a teaching institution, for large, fixed tumors, deep lobe tumors, or recurrent tumors. It has not been proven to reduce facial nerve injury.
Certain intraoperative maneuvers are quite helpful in facilitating the safe conduct of surgery for parotid tumors irrespective of the extent of resection of the gland. An oral endotracheal tube is generally adequate for routine parotidectomy. However, the extra excursion of the jaw that is possible with the use of a nasotracheal tube can be very helpful during surgery for tumors of the deep lobe of the parotid. Precise dissection of the facial nerve requires a bloodless field. In addition to hypotensive anesthesia during nerve dissection, parotid surgery should be carried out with the patient in a reverse Trendelenburg’s position. Adequate extension of the neck is imperative if neck dissection is planned or anticipated. The face and the neck are draped with a transparent plastic drape to allow monitoring of movement of facial muscles. Communication with the anesthesiologist is crucial. A short-acting muscle relaxant permitting facial muscle motion on stimulation of the facial nerve is important whether or not intraoperative facial nerve monitoring is used. The perception that the eyelids on the side of the procedure need to be monitored during facial nerve dissection can result in exposure of the cornea with potentially disastrous results. A water-soluble eye ointment should be applied, and the cornea must be protected with a corneal shield, or the eyelids can be loosely taped shut with clear tape.
Superficial Parotidectomy
Superficial parotidectomy can be defined as excision of parotid tissue that lies lateral to the plane of the facial nerve and its branches. Most benign and malignant parotid tumors can be adequately treated with this procedure. Obviously, patients who have clinical evidence of infiltration of the facial nerve are not suitable for this operation.
No specific preoperative preparation is necessary, but patients must be counseled about complications such as temporary and permanent damage to the facial nerve, numbness, gustatory sweating, seroma, hematoma, and cosmetic changes.
The skin incision for superificial parotidectomy shown in Fig. 15–2 is designed to provide adequate exposure for surgical resection and optimal healing for a good cosmetic outcome. A suitable preauricular skin crease is used in older patients, and the incision then curves around the lobule of the ear to turn anteriorly along an appropriately located upper neck skin crease. If a preauricular skin crease is not easily identifiable, especially in young patients, the upper part of the incision is placed just inside the free anterior border of the tragus of the ear (Fig. 15–3). The preauricular skin incision is taken, and the skin is carefully elevated off the tragal cartilage using fine skin hooks and a no. 15 scalpel blade. Alternatively, the use of a fine needle tip electrocautery for the skin incision leads to a postoperative scar comparable to scalpel dissection and simultaneously provides initial hemostasis. Because of the heightened risk to the facial nerve, aggressive use of electrosurgical instrumentation is otherwise eschewed. The anterior skin flap is elevated, keeping the plane of dissection between the subcutaneous fat on the undersurface of the flap and the dense parotid fascia (Fig. 15–4).
As dissection proceeds anteriorly and past the angle of the mandible, the uppermost fibers of the platysma muscle are divided in the direction of the skin incision, and the flap is now elevated just deep to the platysma. Dissection of the flap with a fine mosquito hemostat, bipolar cautery, and plastic scissors is an atraumatic, hemostatic technique that is suggested. New and alternative dissection techniques include laser-cutting technologies, ultrasound scalpels,14 water-jet dissection, and diathermy scissors.15 The peripheral branches of the facial nerve exit the anterior aspect of the parotid gland and are liable to injury during this phase of the operation. The skin flap is now dissected posteriorly to expose the cartilaginous auditory canal, the mastoid tip, and the upper portion of the sternocleidomastoid muscle. At this stage, elevation of the skin flaps for surgical exposure is complete, and the next phase of the operation is aimed at displaying anatomical landmarks in preparation for identification of the main trunk of the facial nerve. Magnification can be helpful for this portion of the procedure. The fascia covering the muscle is incised parallel to its anterior border to retract the muscle posteriorly and the adjacent parotid tissue anteriorly (Fig. 15–5).
FIGURE 15-2 The incision follows skin creases in the preauricular region and the upper neck.
The greater auricular nerve runs along the upper portion of the sternocleidomastoid muscle to enter the superficial portion of the parotid gland on its way to the overlying skin. Preservation of the posterior branches of the greater auricular nerve, without compromising the surgical exposure or risk of recurrence, is possible in many cases. This will result in potentially diminished ear lobular and angle of the mandible numbness postoperatively. The greater auricular nerve, however, occasionally needs to be sectioned in order to mobilize the parotid gland off the sternocleidomastoid muscle. Fortunately, most patients do not report significant symptoms associated with sacrifice of this nerve.16 It is good practice to divide the greater auricular nerve close to the lower border of the parotid gland, keeping well clear of any tumor in order to preserve as much length as possible in case the need for cable grafting arises. The posterior belly of the digastric muscle can now be displayed by dissecting off the parotid tissue from the sternocleidomastoid muscle (Fig. 15–6). Dissection continues in a cephalad direction in the plane between the auditory canal and the posterior aspect of the parotid gland. This final preparatory step for dissection and identification of the main trunk of the facial nerve must be undertaken with great caution to avoid inadvertent injury to the facial nerve. Careful blunt dissection with a fine hemostat and judicious use of the bipolar cautery allow the surgeon to clearly delineate the posterior belly of the digastric muscle all the way to its insertion into the digastric groove of the mastoid process.
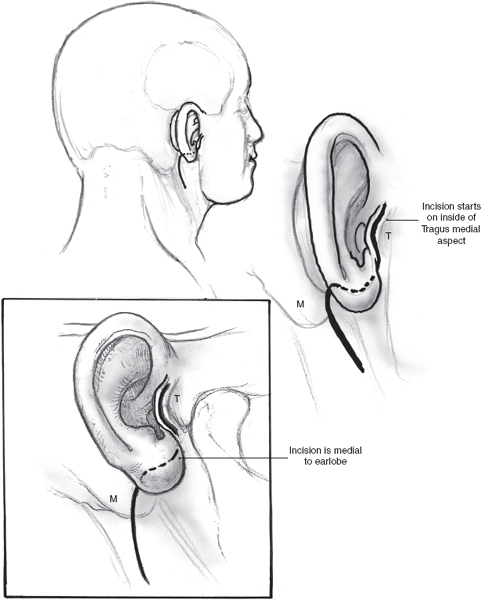
FIGURE 15-3 Modification of the parotidectomy incision in young patients. T, tragus; M, mastoid process.
Two deep right-angled retractors are used to gently retract the parotid gland in an anterior direction so that the parotid tissue overlying the main trunk of the facial nerve is put on stretch (Fig. 15–7). At this stage it is useful to review the regional anatomical landmarks that aid the safe identification of the main trunk of the facial nerve (see Chapter 1, Fig. 1–5). The main trunk of the facial nerve enters the parotid gland high on its posteromedial surface, and its branches travel in an oblique plane to surface at the anterior border of the gland. Certain anatomical structures in the region can be relied upon to guide the dissection of the nerve. The main trunk of the nerve is situated in the notch formed by the superior edge of the posterior belly of the digastric muscle, the anterior border of the mastoid tip, and the inferior border of the auditory canal. A long, curved hemostat is used to gently spread the tissue in this region along the direction of the nerve (Fig. 15–8). This part of the dissection is best carried out in small increments, ensuring absolute hemostasis before the next tissue plane is dissected. As a general rule, a small blood vessel that runs parallel and immediately superficial to the nerve serves to alert the surgeon of its proximity.
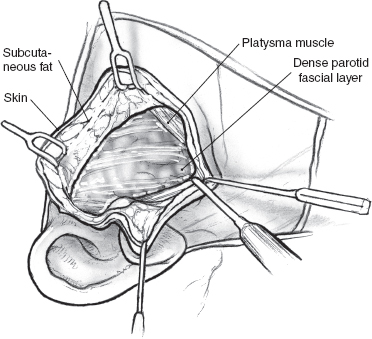
FIGURE 15-4 Elevation of the anterior skin flap.
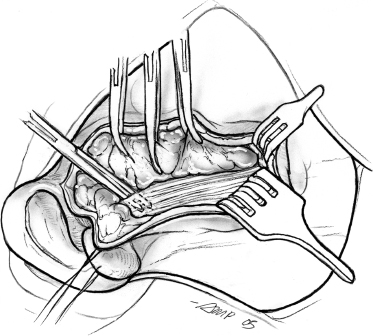
FIGURE 15-5 Dissection of the anterior border of the sternocleidomastoid muscle.
An alternative approach to identification of the facial nerve is utilizing the tympanomastoid suture (see Fig. 1–6). This can provide for the experienced surgeon a single landmark approach to the parotid. The tragal pointer has a blunt and variable tip that can change with retraction and lies 1 to 2 cm from the facial nerve.17,18 It only points to the facial nerve in 20% of cases.18 The styloid process is deep to the facial nerve and if used to identify the facial nerve risks injury to this nerve. The tympanomastoid suture provides the advantage over other anatomical landmarks in being a fixed landmark with less variability as can be seen with a muscle landmark such as the posterior belly of the digastric. It is a closer anatomical landmark to the facial nerve compared with the posterior belly of the digastric muscle. The tympanomastoid suture is invariably 1 to 3 mm inferior to the pes anserinus of the facial nerve in comparison with 0.5 to 1.5 cm for the posterior belly of the digastric muscle. Some surgeons are uncomfortable with this landmark because it is a palpated and not a visualized landmark like the posterior belly of the digastric muscle.
The retrograde approach to identification of the facial nerve utilizes the retromandibular and/or superficial temporal veins in exposing the marginal mandibularis branch and temporalis branches of the facial nerve, respectively.19,20 The retrograde approach has largely lost favor because of the variability of the peripheral facial nerve branches including their position at times deep to the venous structures.21 Most variations of the facial nerve occur distal to the bifurcation of the main trunk of the facial nerve, distal to the pes anserinus. The main trunk is the most consistent portion of the nerve.22 The retrograde approach continues to be advocated for revision surgery where scar tissue impedes dissection of the facial nerve and for large, bulky tumors that may obstruct visualization of the main facial nerve trunk.
After the main trunk of the facial nerve has been clearly identified, the objective of the next part of the procedure is to dissect its branches and in the process excise the superficial lobe of the parotid gland along with the tumor. This phase of the procedure requires adequate exposure and absolute hemostasis for its safe conduct. It must be reiterated here that the patient should be in a reverse Trendelenburg’s position, and the anesthesiologist must provide hypotensive anesthesia maintaining the systolic blood pressure around 90 mmHg. The tip of a long, curved hemostat is inserted along the direction of the nerve being dissected, between its superficial surface and the overlying parotid tissue (Fig. 15–8). Troublesome bleeding can increase the risk of inadvertent damage to the nerve if the plane of dissection is not maintained directly on the surface of the nerve being dissected. The tip of the hemostat is then lifted up away from the underlying nerve, and the bridge of parotid tissue between the prongs is sharply divided with Reynold’s scissors. Bleeding from fine vessels in the divided parotid gland is controlled with bipolar coagulation. Bigger vessels are clamped using fine-tip hemostats and ligated carefully with 4.0 chromic catgut. The main trunk of the nerve is dissected in this manner until its bifurcation becomes visible. Although the main trunk is usually 1 to 3 cm in length, it may be longer if the nerve has been put to stretch by an underlying tumor. The dissection now proceeds in this plane superficial to the nerve so that its divisions and branches are clearly identified. In terms of the sequence of dissection of the divisions of the nerve, dissection of the tumor-bearing half of the superficial lobe is generally completed after the normal gland has been dissected. As the dissection proceeds toward the periphery of the parotid gland, it is important to remember that the nerves run in an oblique deep-to-superficial plane in the posterior-to-anterior direction so that the fine, peripheral branches are at risk of injury. This is especially true for the buccal branches that course parallel to Stensen’s duct in the region of the cheek. The duct is carefully dissected free from any adjoining nerves, divided sharply, and its stump is ligated with 3-0 chromic catgut (Fig. 15–9).
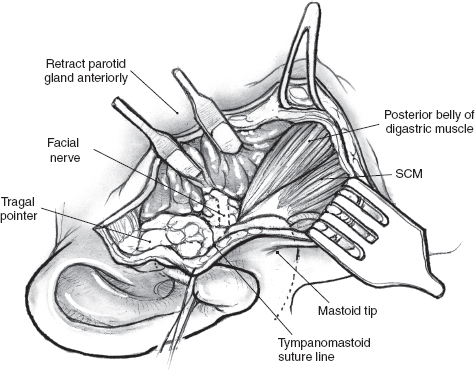
FIGURE 15-6 The posterior belly of the digastric muscle can be displayed by dissecting the posterior border of the parotid gland from the sternocleidomastoid (SCM) muscle.
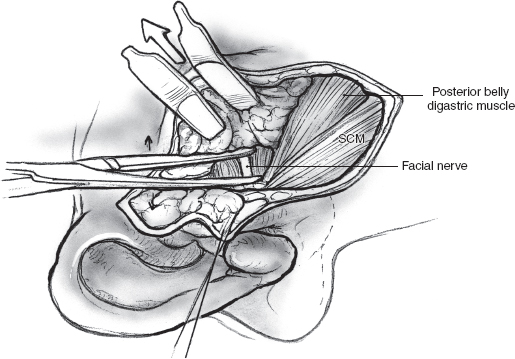
FIGURE 15-7 Gentle retraction with two right-angled retractors provides surgical exposure for dissection of the facial nerve. SCM, sternocleidomastoid muscle.
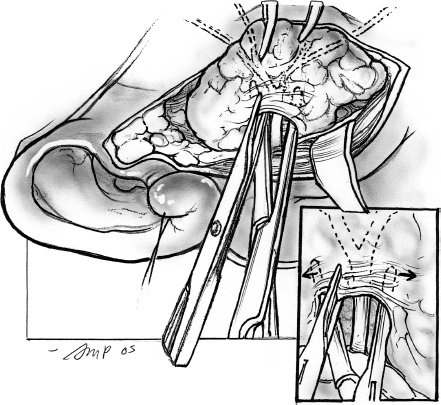
FIGURE 15-8 Dissection of the branches of the facial nerve.
After delivering the specimen, complete hemostasis must be secured. It is important to reverse hypotension before proceeding to close the surgical wound. Drainage of the surgical bed is achieved by a Penrose drain or closed suction drainage. Closed suction drainage should ensure the tip of the drain is secured away from any branches of the dissected facial nerve. A closed suction drain coupled with absorbable packing over the surgical bed can obviate the need for a compression dressing, providing surveillance for hematoma, and can invariably be removed the following day without risk to the facial nerve. The wound is irrigated with dilute antibiotic solution in saline, and hemostasis is confirmed. The incision is then closed in two layers with interrupted, inverting 3-0 chromic catgut sutures to approximate the subcutaneous tissue, and the skin is closed with interrupted 4-0 nylon sutures or an absorbable subcuticular suture. The suture line is cleaned, and a light film of antibiotic ointment is applied. If a Penrose drain is used, the wound is covered with light dressing held in place with tube gauze. A suction drain does not require a dressing. The patient requires no special postoperative care, and the Penrose drain or suction drain is removed the next day as soon as the discharge tapers out.
Complications of parotidectomy are listed in Table 15-2 The most dreaded complication is loss of function of the facial nerve or its branches. Management of this problem is difficult and is discussed in detail in Chapter 16. Temporary functional derangement, including paresis, is not infrequent and is generally unpredictable. However, temporary paresis may be expected when the tumor has been dissected off the nerve. In the absence of overt injury to the nerve, recovery of function generally occurs within 3 months.
Secondary hemorrhage and hematoma formation are often preventible complications. Once the need for surgical exploration of the wound and evacuation of the hematoma is determined, the procedure must be undertaken in the operating room under general anesthetic. It is useful to ensure that the patient’s blood pressure is in the normal preoperative range while the wound is being explored. The entire incision must be opened to provide maximal exposure, and it must be emphasized that attempts at limited exploration can result in injury to the facial nerve. After evacuating the hematoma, copious amounts of warm saline are used to irrigate the wound. The greatest caution must be exercised to avoid injuring the facial nerve or its branches during evacuation of the hematoma and attempts at hemostasis. The surgical team must resist the temptation to use the suction cannula in the region of the parotid bed during the exploration. The offending vessel is generally located on the cut surface of the remnant parotid tissue or may be a branch of the retromandibular vein. Before hemostasis is attempted, the relationship of any bleeding vessel to the facial nerve branches must be precisely identified. Fine-tipped micro-hemostats are used, and the bleeding vessel must be carefully ligated. After ensuring hemostasis, the incision is reapproximated in the usual fashion over a Penrose or suction drain.
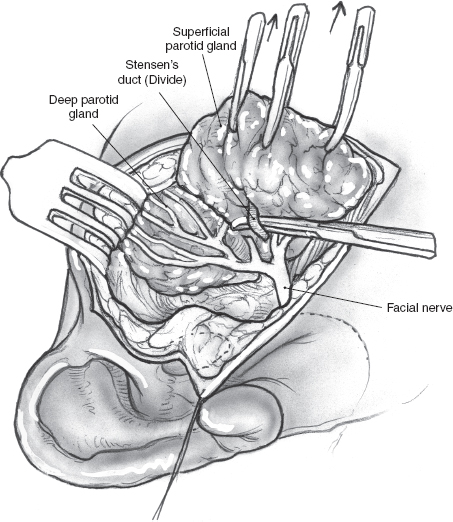
FIGURE 15-9 Dissection and division of Stensen’s duct.
Sialoceles can occur after parotid surgery and can also be seen after penetrating parotid injury. A sialocele is a discrete collection of saliva, either within a duct (and thus here represents a retention cyst) or after trauma or other disruption of the gland (and here is not present in an epithelial-lined space but is rimmed by an inflammatory reaction, a pseudocyst). Treatment is almost always effective with serial aspiration or even observation alone. Resolution occurs in 4 to 6 weeks. Antibiotics covering Staphylococcus aureus can be used in the initial time period. An external salivary fistula can occasionally develop, which also usually resolves spontaneously. The very rare resistant case has been treated with oral anticholinergics, tympanic neurectomy, and more recently botulinum toxin.23
Numbness after parotidectomy generally recedes after 6 months. Even with preservation of the posterior branch of the greater auricular nerve, patients can still have lobular and angle of mandible skin numbness and must be thus advised.
Reconstruction of the postparotidectomy defect can be performed using AlloDerm (Life Cell, The Woodlands, TX).24 An inferiorly based platysma muscle—cervical fascia—sternocleidomastoid muscle flap25 or sternocleidomastoid muscle transposition flap after parotidectomy can also be used to improve the cosmetic result.26 In the latter procedure the muscle must be freed from its mastoid and skull base attachments with inferior dissection until rotation of the flap is possible to reach the zygomatic arch superiorly and masseter muscle anteriorly, where it is sutured.27 This technique, like other interposition flaps, will not prevent Frey’s syndrome. Its use must be balanced with the significant risk of masking recurrent disease, additional operative time, trauma to the greater auricular nerve, and the observation that many patients will not complain of the defect even after total parotidectomy.27
Table 15-2 The Complications of Superficial Protidectomy
| Acute | Paresis or paralysis of facial nerve or its branches |
| Bleeding or hematoma | |
| Seroma | |
| Salivary fistula | |
| Late | Sensory deficits |
| Cosmetic deformity | |
| Frey’s syndrome |
Frey’s syndrome is another late complication of parotid surgery. Lucie Frey, whose patient was a Polish soldier treated for a typhoid abscess of the parotid gland, published her classic report of gustatory sweating in 1923.28 Frey’s syndrome (gustatory sweating) results from abnormal neural connections between parasympathetic cholinergic nerve fibers of the parotid gland, with severed sympathetic cholinergic receptors innervating sweat glands and vessels of the face, and manifests as gustatory sweating, facial flushing, a sensation of warmth over the preauricular and temporal area, and piloerection. Most cases develop from parotid surgery, although neck dissection, thoracocervical sympathectomies, and submandibular gland resection can also cause Frey’s syndrome.29 Frey’s syndrome is objectively assessed by Minor’s starch-iodine test, where a solution of iodine, castor oil, and absolute alcohol is applied to a patient’s cheek and then dusted with starch powder. Almost all patients subjected to Minor’s starch-iodine test will exhibit signs of Frey’s syndrome, but only 15 to 25% report subjective symptoms to their physician.30,31 Infrared thermography is a noninvasive test that provides a qualitative visual analysis of the cutaneous capillary response in Frey’s syndrome.32
The incidence of Frey’s syndrome is reportedly dependent on the amount of normal parotid parenchyma dissected: total parotidectomy (47%), superficial parotidectomy (17%), and partial superficial parotidectomy (10%).9 Many treatments have been proposed for Frey’s syndrome. Thickness of the flap has not correlated with patient symptoms. Application of topical 20% aluminum chloride solution has met with little success and poor compliance, as has atropine or application of scopolamine hydrobromide ointment before meals. Fat and fascia lata interpositions have failed to reduce gustatory sweating.33 The sternocleidomastoid muscle flap has been promoted to reduce the incidence of Frey’s syndrome in one report, but refuted in another.34,35 Limited success has been achieved with tympanic plexus neurectomy.31 Botulinum toxin is being used increasingly, blocking the release of acetylcholine at the neuromuscular junction and peripheral cholinergic nerve terminals, and preventing neurotransmission.36
Partial Superficial (Limited) Parotidectomy
Selected tumors in certain locations within the parotid gland are easily amenable to partial superficial parotidectomy (see Chapter 9, Fig. 9–7). The surgeon must be able to visualize the relationship of the tumor to the underlying branches of the facial nerve clearly. These relevant branches do need to be identified and preserved during the operation. The surgical operative steps of this limited procedure are therefore identical in principle to superficial parotidectomy, but the dissection is limited to only part of the gland. As in complete superficial parotidectomy, the facial nerve is dissected distally from just beyond the pes anserinus to the proximal offshoots of the nerve branches with a fine plastic hemostat, bipolar cautery, and plastic scissors. If the tumor is located in the tail of the parotid gland, as frequently occurs, the marginal mandibular branch of the facial nerve and the inferior branches of the buccal branches are dissected distally. No further dissection of the frontozygomatic branches is executed beyond the previously mentioned proximal exposure. For tumors located more superiorly, more distal dissection of the marginal mandibular branch is avoided. Two centimeters of normal parotid gland is obtained around the tumor, except where it abuts the facial nerve or superficial fascia. Expected rates of transient facial nerve dysfunction and subjective Frey’s syndrome are 20% and 10%, respectively, with partial superficial parotidectomy.30 Irrespective of the amount of normal parotid tissue resected, the proximity of the deep surface of the tumor to the underlying branches of the facial nerve usually determines the extent of the surgical “margins.” It is important that this concept is used in properly selected patients. In patients with a mixed tumor less than 4 cm in size, mobile, and located in the superficial lobe, the rate of recurrence is no higher with partial superficial parotidectomy, compared with complete superficial parotidectomy.9
Surgery for Tumors of the Deep Lobe of the Parotid Gland
The “deep” lobe of the parotid gland is defined as the parotid tissue that lies medial to the plane of the facial nerve. In terms of volume, this constitutes less than 20% of the salivary tissue, and therefore tumors of this part of the gland are rare. The vast majority of deep lobe tumors are benign, and depending on their location within the gland, they may present in either the retromandibular area or as a parapharyngeal mass. Preoperative histological diagnosis is usually not necessary because radiologic imaging is extremely reliable in differential diagnosis. Image-guided FNAC can be performed, but access can be complicated. Open biopsy is not recommended and, especially if done transorally, may tremendously complicate subsequent definitive excision.
Dissection of deep lobe tumor is accomplished after removal of parotid parenchyma lateral to the facial nerve. The tumor is dissected from branches of the facial nerve with a fine hemostat, bipolar coagulation, and plastic scissors. For the deep lobe tumor, greater areas of exposed capsule can be expected than from dissection of a tumor from the superficial lobe. Careful handling of the deep lobe parotid parenchyma and the capsule of the tumor will reduce the risk of rupture. A higher rate of transient facial nerve dysfunction can be expected.9
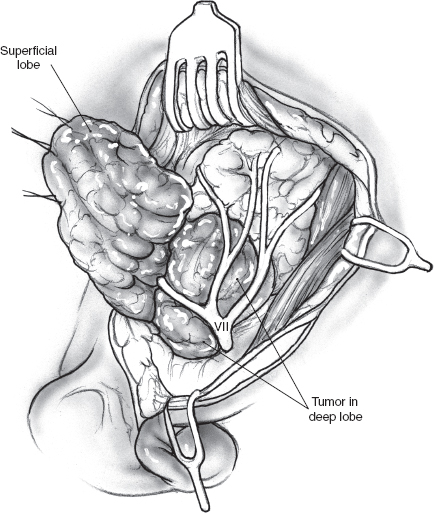
FIGURE 15-10 As the superficial parotidectomy nears completion, the deep lobe tumor is seen deep to the facial nerve and its branches.
Transcervical-Transparotid Approach for Tumors of the Deep Lobe of the Parotid Gland
Deep lobe tumors located in the retromandibular region and most parapharyngeal space tumors are essentially amenable to surgical resection by a transcervical-transparotid approach. The preoperative considerations are the same as described for superficial parotidectomy above, but there are certain important technical variations that may help increase surgical exposure of the deep lobe tumor. Nasotracheal, as compared to the usual oral endotracheal, tube provides an extra few centimeters of excursion of the lower jaw that can be crucial in accessing the tumor. Complete muscle relaxation obviously helps, and this may be requested once superficial parotidectomy and dissection of the facial nerve are complete. Other surgical maneuvers for improving access to the parapharyngeal space will be described below.
The operation commences with a superficial parotidectomy and dissection of the facial nerve. Although a formal superficial parotidectomy may not always seem necessary, it certainly is advisable for adequate exposure of most deep lobe tumors. After completion of the superficial parotidectomy, the tumor of the deep lobe of the parotid gland should be visible stretching the facial nerve and its branches over it (Fig. 15–10). The nerve and its branches are carefully dissected off the underlying tumor using a nerve hook and sharp, fine scissors to minimize trauma. The tumor can now be mobilized all along its periphery using gentle digital dissection combined with careful sharp division of fibrous bands, while the nerve and its branches are gently retracted cephalad (Fig. 15–11). Blunt dissection must be gentle to avoid rupture, and the specimen should be inspected carefully to ensure complete removal of all lobules of the tumor. The surgical bed requires no special reconstruction. After meticulous hemostasis, the incision is closed in layers over a Penrose or suction drain.
Tumors of the deep lobe that grow into the para-pharyngeal space can often present as a submucosal lateral pharyngeal mass that is easily visible through the open mouth (see Chapter 9, Fig. 9–8) It must be emphasized that such a tumor should under no circumstances be approached per orally for either diagnostic biopsy or for surgical resection. Radiologic imaging using CT and/or MRI scans is mandatory, not only because it can reliably exclude other parapharyngeal space tumors, but also because it allows the surgeon a clear perception of the three-dimensional extent of the tumor (see Chapter 2, Figs. 2–13, Figs. 2–14, Figs. 2–15). The combined transcervical-transparotid approach described above is most often adequate for these tumors as well. In addition to the anesthetic considerations described above, certain intraoperative maneuvers can be very useful in enhancing access to the parapharyngeal space. A bone hook can be used to pull the angle of the mandible in an upward direction to improve access between it and the tumor. Although dislocation of the mandible at the temporomandibular joint and forward displacement may help, this maneuver is not easy and is generally not recommended. Division of the posterior belly of the digastric muscle, the stylohyoid complex, and/or the stylomandibular ligament can widen access, but it may affect function postoperatively. Retraction of the sternocleidomastoid muscle and excision of fibrofatty and lymphatic tissue at level II should be standard procedure, but if greater anteroinferior access is required, the submandibular gland can be excised without major sequelae. Digital dissection of the tumor, especially on its superoposterior aspect, cannot be carried out under direct vision. It is therefore crucial that the surgeon be familiar with regional anatomy, including visualization of the internal jugular vein, carotid artery, and cranial nerves (CN) IX, X, XI, and XII (see Chapter 1, Figs. 1–8, Figs. 1–9), and dissect very carefully in the parapharyngeal loose areolar tissue to avoid fracturing the tumor. Any areas of resistance that are felt as fibrous bands must be patiently delineated by measured digital dissection and divided sharply. The specimen must be carefully inspected for complete excision, and closure of the incision is accomplished in the usual fashion over a Penrose or suction drain.
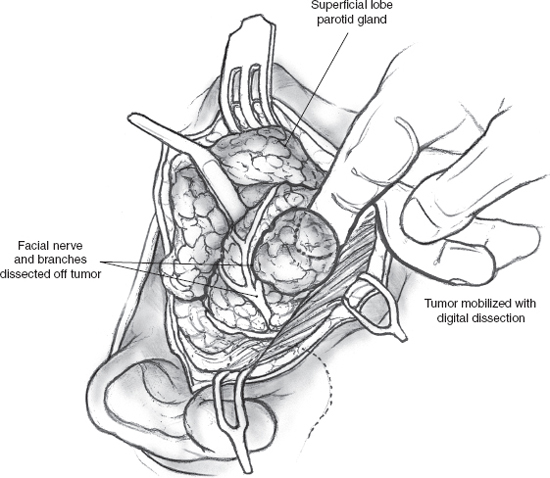
FIGURE 15-11 Dissection of a deep lobe parotid tumor. The facial nerve and its branches are carefully dissected off the tumor, then retracted with nerve hooks, and the tumor is mobilized using gentle digital dissection.
Mandibulotomy Approach for Tumors of the Deep Lobe of the Parotid Gland
The vast majority of tumors of the deep lobe of the parotid gland, even those that are of considerable dimension, are resectable by the transcervical-superficial parotidectomy approach described above. This approach, however, is inadequate and may indeed be hazardous under certain circumstances. The mandibulotomy approach is indicated in situations that require more comprehensive exposure of the parapharyngeal space (Table 15-3).
The mandibulotomy, or mandibular swing, approach has been well described in the literature,37 as have the pros and cons of the various types of mandibular osteotomy.1
Table 15-3 Indications for the Mandibulotomy Approach to Tumors of the Deep Lobe of the Parotid Gland
| Malignant tumor |
| Locally recurrent tumor |
| Previously violated surgical field, especially for attempted peroral resection of a malignant tumor |
| Tumors that cannot be safely removed with the transcervical/ transparotid approach |
The paramedian mandibulotomy is the preferred route of access to the parapharyngeal space for a number of reasons (Table 15-4). The osteotomy is designed to pass through an area of bone between the diverging roots of adjacent teeth in order to avoid the need for dental extraction and also so that the bone cut does not expose the adjacent dental roots. In most patients, the site that fulfills this condition is located between the lateral incisor and canine teeth. For obvious reasons, the site of the osteotomy must be planned preoperatively on a radiograph. Radiographic imaging is also mandatory to avoid placing the osteotomy through pathologic bone, an event that can result in nonunion of the osteotomy with all its attendant complications. Similarly, dental sepsis should be controlled before the operation, or an alternative osteotomy site must be selected. The vertical limb is placed to bisect the space between the lateral incisor and the canine teeth on the side of the tumor, and extends about a centimeter to a level just beyond the tips of the dental roots. This is important to avoid exposure or amputation of the dental roots, both of which will result in nonvital insensate teeth. The osteotomy is then angled down toward the symphysis to create a notch. This notch provides better stability to the healing mandibular segments against shearing forces compared with a straight, vertical osteotomy.
After a nasotracheal anesthetic tube has been placed, the patient is placed in a reverse Trendelenburg’s position, and skin incision for a lower cheek flap approach is outlined (Fig. 15–12). The incision begins in the midline on the lower lip and extends vertically down the midline of the chin and the upper neck up to the level of the hyoid bone, where it turns laterally into an appropriate skin crease. The horizontal limb of the incision continues over the sternocleidomastoid muscle, where it turns upward to continue into the preauricular region as a standard parotidectomy incision.
Table 15-4 The Pros and Cons of the Paramedian Mandibulotomy Approach
| Pros |
| Provides wide exposure to the parapharyngeal space |
| Does not require division of inferior alveolar (preserves sensation to the teeth) or mental nerve (preserves sensation to the chin and lower lip) |
| Requires division of only the mylohyoid muscle with minimal disruption of the swallowing mechanism and the stabilizing muscular forces of the muscles of mastication |
| Does not need extraction of any teeth |
| Postoperative care is easier because the suture line is located anteriorly in the mouth. |
| The osteotomy and hardware used to fix it do not interfere with any portals of radiation therapy that may be necessary for adjuvant treatment of the tumor. |
| Cons |
| Technically more demanding |
| Delayed union or nonunion can cause significant pain and morbidity. |
The first stage of the operation consists of a superficial parotidectomy, with identification and preservation of the facial nerve and its branches as described above. Appropriate dissection of the cervical nodes is then completed if indicated.
The next phase involves exposure of the mandible for a paramedian mandibulotomy through a lower cheek flap. The lower lip is incised sharply and cleanly in the midline, and the full thickness of the lip and the chin are then divided down to a point about 0.5 cm away from the reflection of the mucosa in the gingivobuccal sulcus. The mucosal incision is turned to the side away from the tumor for approximately 2 cm, so that a limited lower cheek flap may be elevated on that side. The soft tissue over the mandible is then elevated in a plane just superficial to the periosteum, taking care to stop well short of the mental foramen where the mental nerve exits. The proposed osteotomy site, medial to the mental foramen, is marked out, and the periosteum of the mandible is incised along it. Two titanium mini-plates are contoured to straddle the osteotomy, one on the anterior surface of the mandible and the other along its inferior border. Drill holes are made to prelocalize these mini-plates that are then stored away for later use (Figs. 15–13, 15–14). An important consideration in positioning the mini-plates and placing drill holes is to avoid injuring the dental roots. The osteotomy is now completed using a fine blade on a high-speed power saw. The vertical limb is taken first, starting from the upper border of the lower gum in the space between the lateral incisor and the canine teeth. The angled limb of the osteotomy is started from the lower border of the mandible, and as the blade of the saw approaches the vertical limb, great care must be taken to avoid overriding it. A gentle tap with a fine osteotome completes the osteotomy. Bleeding from the cut ends of the mandible is controlled using bone wax.
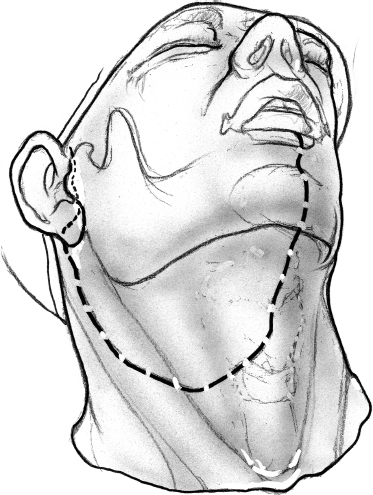
FIGURE 15-12 Outline of the incision for lower cheek flap approach.
The mandibular segments are distracted with loop retractors, and the mucosa of the floor of the mouth on the side of the tumor is incised. The hypoglossal nerve is observed and preserved. The mucosal incision starts anteriorly at the site of the osteotomy and is continued into the floor of the mouth, so that a cuff of mucosa about 1 cm wide is left attached to the lower gum (Fig. 15–15). An adequate cuff of attached mucosa allows safe closure of the floor of the mouth at the conclusion of the procedure. The incision in the floor of the mouth is continued posteriorly up to the anterior tonsillar pillar, progressively retracting the divided mandible laterally. Any further extension of the incision will require division of the lingual nerve as it crosses over to enter the tongue. The mucosal incision can also be modified posteriorly to include a portion of the lateral pharyngeal wall that may be adherent to underlying tumor, either by direct infiltration or more commonly as a result of adhesion from a previous attempt at peroral biopsy or excision (Fig. 15–15). As dissection proceeds, the tongue is pulled anteriorly and away from the tumor, and division of the mylohyoid muscle allows wide retraction of the mandibular segments to provide adequate exposure to the para-pharyngeal space (Fig. 15–16).
The facial nerve and its branches are carefully elevated off the underlying deep lobe tumor and retracted cephalad. As described before, gentle digital dissection is used to mobilize the tumor laterally, while its medial and superior aspects can be safely dissected via the exposure afforded by the mandibulotomy. Circumferential mobilization of the tumor is thus completed to ensure adequate monobloc excision, and the tumor is delivered. If a significant amount of lateral pharyngeal wall mucosa needed resection, reconstruction using either a pectoralis major myocutaneous flap or a microvascular free flap becomes necessary. This amplifies the magnitude of the procedure several-fold, and hence the need to reemphasize that any peroral attempt at biopsy or excision of these tumors is absolutely contraindicated.
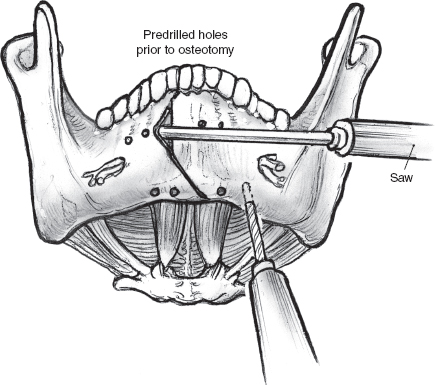
FIGURE 15-13 The site of mandibulotomy is marked on the mandible, and holes are drilled prior to mandibulotomy.
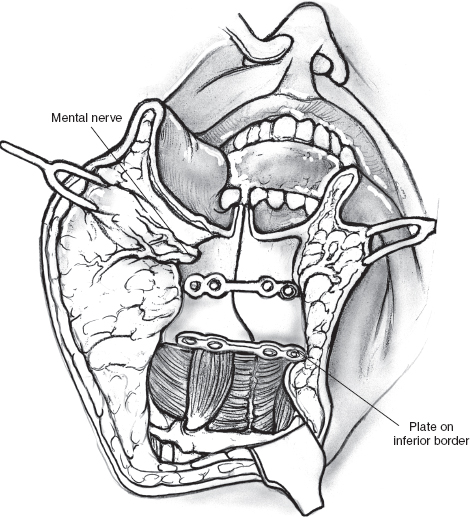
FIGURE 15-14 Mini-plates are prelocalized prior to the osteotomy. Diagram shows accurate approximation of the mandibulotomy using contoured mini-plates and screws.
After ensuring hemostasis, a nasogastric feeding tube is inserted and secured in place. The incision in the mucosa of the floor of the mouth is repaired with interrupted chromic catgut sutures starting posteriorly and proceeding in an anterior direction. The contoured mini-plates and screws of appropriate length are now used to secure the mandibular segments in accurate approximation at the site of the osteotomy (Fig. 15–14). Closure of the lower lip incision and cheek flap can now begin. A 5-0 nylon suture is taken to accurately align the vermilion border of the lower lip. This suture is held long and used to guide subsequent closure of the mucosa of the lower lip and the gingivolabial sulcus. The skeletal muscle and the mucosa of the lip are approximated in two separate layers with interrupted 3-0 chromic catgut sutures starting at the vermilion and working toward the lower gum. Before closure of the rest of the incision, the divided stumps of the mylohyoid muscle are sutured with chromic catgut sutures. Two closed-suction drainage tubes are inserted into the neck, and the remainder of the incision is closed in two layers. It is important to ensure that the suction drains are placed away from the facial nerve and its branches.
Postoperatively, the patient is fed through the nasogastric tube for about a week. If a trial of puréed food is successful, the patient’s intake can be gradually advanced to a soft diet over the ensuing days. The nasogastric tube is removed when the patient’s oral intake is able to meet the daily nutritional requirement. Meticulous oral hygiene is essential to good healing and avoiding sepsis at the osteotomy. As healing progresses, the patient can gradually return to a normal diet, and the mandibulotomy should eventually result in no dietary restrictions. The lip and neck incisions also heal with minimal cosmetic deformity.
Resection of an Accessory Parotid Tumor
Accessory parotid tissue is most commonly located anterior to the parotid gland, along the course of Stensen’s duct. Accessory parotid glands have been described to be either round or triangular in shape and almost always contain anastomoses of the zygomatic and buccal branches of the facial nerve within their substance. Although this tissue is susceptible to most salivary pathology, tumors of the accessory parotid glands are more frequently malignant than those of the parotid glands themselves.
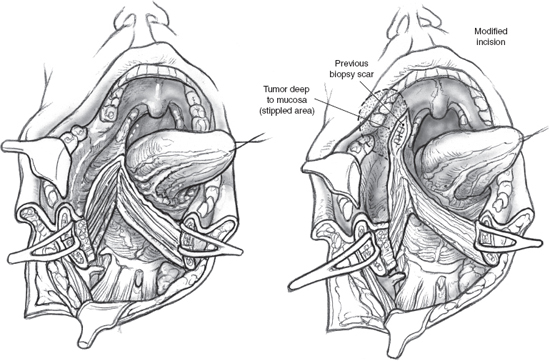
FIGURE 15-15 The mucosa of the floor of the mouth is incised to preserve a 1 cm wide cuff of mucosa attached to the lower gum. The mucosal incision can be modified to include a portion of the lateral pharyngeal wall mucosa that may be adherent to underlying tumor.
The patient with a tumor of the accessory parotid gland generally presents with a firm, well-defined mass in the cheek. Clinical examination of the mass should provide the surgeon a good idea about its anatomical extent, but imaging can be very helpful in delineating its relationships to the parotid gland and the masseter muscle. Although a formal superficial parotidectomy with dissection of all the branches of the facial nerve is a good policy, more limited dissections are appropriate for smaller tumors, provided the surgeon recognizes the importance of meticulous dissection of the branches of the nerve in proximity of the tumor.
Surgical resection of an accessory parotid tumor requires a greater degree of surgical exposure of the cheek compared with a superficial parotidectomy. Any attempts at limiting the skin incision, or worse, placing it directly over the tumor can only increase the risk of nerve injury and should be discouraged. Because the tumor is located anteriorly in the cheek, surgical exposure requires a longer cheek flap. The standard parotidectomy incision is modified as shown in Fig. 15–17. The transverse extension toward the temple should be taken with great caution after the temporal and zygomatic branches of the facial nerve have been identified. The anterior skin flap is elevated to obtain adequate exposure of the tumor. Safe conduct of the operation depends on identification of the peripheral branches of the facial nerve. An experienced surgeon should be able to dissect the nerve branches as they exit the parotid gland without any difficulty, but it is always safe to perform a superficial parotidectomy so that the relevant branches can be identified as they come off the main divisions of the nerve. Superficial parotidectomy is also indicated if the tumor abuts the substance of the parotid gland and is malignant or if the Stensen’s duct has to be resected with the tumor. The branches of the facial nerve and Stensen’s duct are situated in close proximity to the accessory parotid tumor. Identification and dissection of Stensen’s duct may be easier if its mucosal opening has been cannulated and a probe has been left in place at the start of the procedure. The branches of the facial nerve are carefully dissected, and the tumor is excised. The incision is closed in layers as described above after superficial parotidectomy.
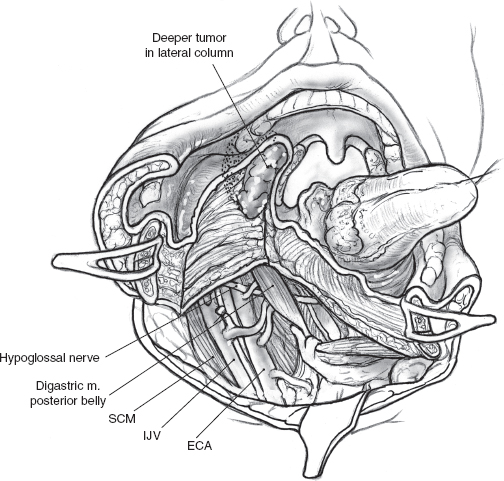
FIGURE 15-16 Division of the mylohyoid muscle allows wide retraction of the mandibular segments and provides adequate exposure of the para-pharyngeal space. ECA, external carotid artery; IJV, interal jugular vein; SCM, sternocleidomastoid muscle.
Management of Recurrent Tumor of the Parotid Gland
Although local recurrence is relatively rare following parotidectomy for mixed tumor of the parotid gland, surgical management of this situation can be problematic. The major concern in undertaking surgery for these patients is obviously the increased risk of injury to the facial nerve. If the tumor recurs after enucleation, surgical resection is relatively straightforward because the tissue planes around the branches of the facial nerve remain intact. However, the likelihood of nerve injury is appreciably higher in the patient who develops multifocal recurrence in the surgical bed following superficial parotidectomy and complete dissection of the facial nerve. Multifocal recurrence often requires treatment of the preauricular skin (Fig. 15–18A, B). The skin defect can usually be reconstructed using a local flap, but more extensive reconstruction such as a microvascular free flap may become necessary for larger defects. The operation begins as usual by entering and often excising the preauricular and upper neck incisions. The facial nerve and its branches are immediately deep to the skin owing to previous superficial parotidectomy, and patient, meticulous dissection is imperative to nerve preservation. The use of a nerve integrity monitor is recommended in this situation, and magnification of the surgical field can also be helpful. If there is significant fibrosis around the anatomical location of the main trunk of the facial nerve, a retrograde approach can be selected. The anterior aspect of the skin incision is excised, and an anterior skin flap is developed to identify the peripheral branches of the facial nerve if a retrograde dissection is selected. With this technique peripheral branches are dissected toward the main trunk of the nerve, resecting the overlying skin and recurrent tumor nodules in the process. The posteriorly based cervical flap is elevated in a plane deep to the platysma muscle and sutured in place over a Penrose drain or suction drain (Fig. 15–19). (Refer to Chapter 9 for a discussion of recurrent mixed tumor.)
Radical Parotidectomy with Resection of the Facial Nerve
Deliberate sacrifice of the facial nerve or its branches is clearly indicated if the patient’s tumor has resulted in clinically evident nerve deficit. Under all other circumstances, the decision to section the nerve is made intraoperatively and is based on a number of interrelated factors. Attempts to salvage a nerve by splitting a tumor that encases it, even if it is benign, are ill advised as local recurrence is guaranteed. However, there is no justification for sacrificing a functioning nerve to provide a “margin” for resection of a tumor that abuts but does not actually involve the nerve. In cases of locoregionally extensive tumor, the morbidity of facial nerve resection cannot be justified if the surgeon is unable to completely clear tumor from other adjacent structures.
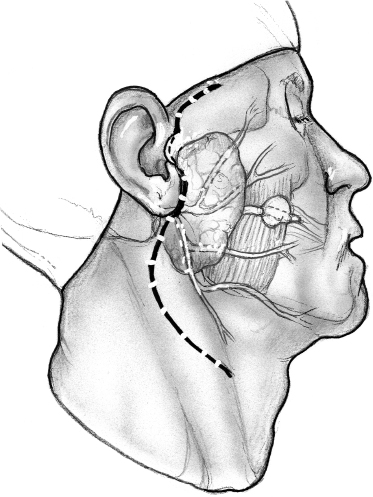
FIGURE 15-17 Modification of the superficial parotidectomy incision for resection of an accessory parotid tumor.
Fig. 15–20 depicts a patient with a locally recurrent tumor following superficial parotidectomy. Radiologic imaging is helpful in this situation to assess the three-dimensional extent of the tumor. As the decision to sacrifice the involved branches of the facial nerve is made intraoperatively, it is important that the patient agree with this plan of action, and the surgical consent should cover this possibility. The surgical team should have the appropriate operating room setup for nerve reconstruction. The indications and technique of nerve grafting are described in detail in Chapter 16.
Surgical access may be through the scar of a previous parotidectomy, which should be excised. It may be difficult to identify and preserve any meaningful length of great auricular nerve in this setting, but this should always be the aim, especially in patients who have not had previous parotidectomy. After the anterior skin flap has been elevated, the main trunk of the facial nerve must be identified even if facial nerve sacrifice has been planned preoperatively. Obviously, the ease with which this is possible and the length of main trunk that can be preserved depend on its relation to recurrent tumor, and every effort is made to maximize the length of normal nerve without compromising tumor excision. The branches of the nerve that are uninvolved and not in immediate proximity to the tumor can be preserved intact depending on the location of the tumor. In the patient depicted in Fig. 15–21, the configuration of the recurrent tumor allows preservation of the upper division along with its temporal and zygomatic branches. The lower division, however, is sectioned proximal to the tumor, and the margin of its stump is examined intraoperatively for tumor involvement. It should be noted that a histologically “negative” nerve margin is by no means indicative of the absence of perineural spread that is well known to skip areas of normal nerve, especially in adenoid cystic carcinoma. As resection of the tumor proceeds, the peripheral branches of the lower division are identified and divided at a safe distance anterior to the tumor. Margins of excision from these branches are examined to rule out the presence of tumor, and their stumps are very carefully tagged with fine silk sutures so that they can be easily identified during the nerve graft procedure. The deep margin of the resection extends to include fibers of the masseter muscle and the periosteum at the angle of the mandible (Fig. 15–21). After nerve grafting is complete, the incision is closed in the usual fashion over a Penrose or suction drain. The postoperative care and results of facial nerve section and reconstruction are considered in detail in Chapter 16.
Radical Parotidectomy with Sleeve Resection of the External Auditory Canal
The external auditory canal lies in close proximity to the parotid gland and is at risk of invasion by aggressive tumors. This situation, however, is more likely in patients who have recurred following previous surgery and radiation therapy. If the tumor has perforated into the auditory canal but does not extend to involve the external ear or the bony external auditory canal, a sleeve resection of the cartilaginous external auditory canal can be performed. Obviously, the operation should not be contemplated without detailed radiographic assessment of the region and determining the local extent of the tumor in relation to the external auditory canal. The tumor is almost always locally extensive and involves multiple structures including the facial nerve by virtue of the regional anatomy. Adequate surgical resection of such a tumor requires radical parotidectomy with resection of skin, external auditory canal, ascending ramus of mandible, and a comprehensive neck dissection. As discussed above, deliberate sacrifice of a functioning nerve can create a therapeutic dilemma, and informed participation of the patient is crucial to successful outcome. A functioning facial nerve is rare in this situation, and complete surgical resection is unlikely without its sacrifice.
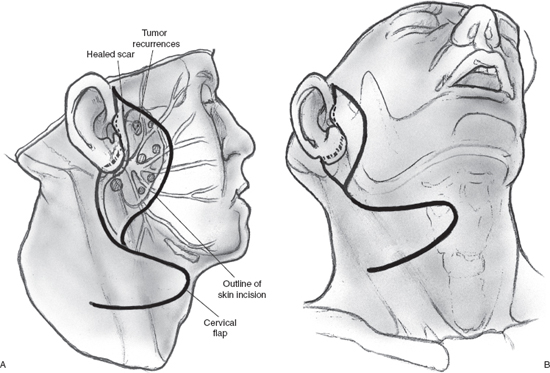
FIGURE 15-18 Multifocal tumor recurrence in the preauricular region following superficial parotidectomy. An area of skin will have to be excised and reconstructed using a posterior cervical flap.
The skin incisions are designed to encompass the involved preauricular skin along with the scar of any previous surgery. The preauricular skin will need to be sacrificed not only because of tumor involvement but also because of decreased viability following previous surgery and radiation therapy. An S-shaped vertical incision is dropped down the neck to provide access for neck dissection. The preauricular skin crease incision begins in the temple as shown and curves around the lobule of the ear to continue posteriorly, so that this superiorly based flap can be elevated with an intact vascular supply to the pinna (Fig. 15–22). A comprehensive neck dissection is completed in an inferior to superior direction, so that the contents of the neck remain attached to the mastoid process.
Resection of the primary tumor now begins with division of the body of the mandible, so that an uninvolved plane of tissue can be dissected deep to the tumor. The ascending ramus of the mandible is divided without breaching the mucosa of the oral cavity. The divided ramus is held in a bone forceps and rotated laterally, so that the pterygoid muscles on its medial aspect can be divided. The pterygoids must be sectioned carefully after careful palpation to obtain an adequate margin of normal muscle. Dissection continues superiorly to divide the zygomatic arch and the temporalis muscle at the level of the arch. This completes the anterior dissection, and the pre-and postauricular skin incisions are now taken to elevate a superiorly based flap containing the pinna. With the external ear lifted up along with the flap, the cartilaginous external auditory canal comes into view. The cartilage of the canal is divided sharply with a knife close to the external ear and proximal to the tumor, maintaining a good margin from it. This releases the pinna, and the superior flap can now be elevated to expose the mastoid and temporal regions. A radical mastoidectomy is completed, and the cartilaginous canal is divided distal to the perforating tumor at its junction, with the bony external auditory canal leaving a cuff of normal external auditory canal skin attached to the canal. The internal jugular vein is dissected, ligated, and divided at the level of the jugular foramen. At this point, the specimen can be delivered by incising the capsule of the temporomandibular joint and disarticulating the mandibular head. After hemostasis is secured, the remnant cartilaginous external auditory canal is sutured to the skin around the remaining bony external auditory canal. Thus a sleeve resection of the external auditory canal is achieved without compromising the pinna or the middle ear. The repaired external auditory canal is packed with Xeroform gauze. The preauricular surgical defect will need reconstruction with either a pectoralis major myocutaneous flap or a free rectus abdominis flap. The neck incision is closed primarily in layers over closed suction drains.
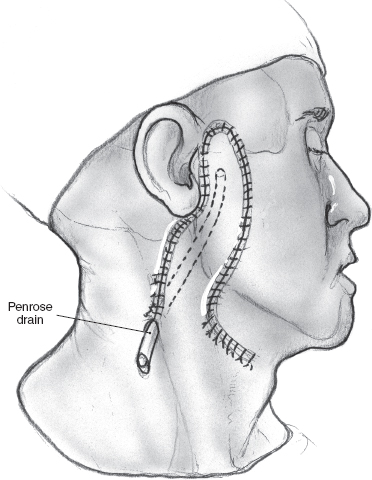
FIGURE 15-19 The cervical rotation flap is used to reconstruct the surgical defect.
Temporal Bone Resection and Management of Skull Base Invasion
Locally extensive tumors of the parotid gland can extend to involve one or more of the numerous anatomical structures of importance in the vicinity. Surgical treatment of these tumors may require resection of part or whole of the temporal bone. Involvement of the skull base of the middle cranial fossa complicates the surgical management even further. It must be emphasized that successful outcome after temporal bone resection or combined craniofacial resection depends on close multidisciplinary cooperation apart from meticulous surgical technique. The reader is referred to a specialist atlas for more detailed description of these procedures.1,38
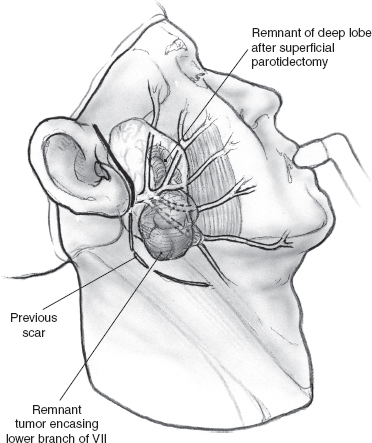
FIGURE 15-20 A recurrent tumor of the parotid gland that encases the branches of the lower division of the facial nerve.
 Surgery of the Submandibular Salivary Gland
Surgery of the Submandibular Salivary Gland
Chronic obstructive and inflammatory pathology frequently affect the submandibular gland. Conservative measures usually produce symptomatic relief, and successful extraction of offending stones may cure the problem before it becomes chronic. A fair proportion of patients, however, will experience multiple inflammatory episodes and require excision of the chronically inflamed gland. The other common indication for surgery includes neoplastic disease, which is more frequently malignant in the submandibular gland compared with the parotid gland. Preoperative differential diagnosis is not easy in the absence of overt signs of malignant behavior. As discussed above, the role of FNAC remains unclear because an aspirate negative for malignant cells cannot rule out malignancy with certainty. Similarly, cross-sectional imaging generally does not add much to the clinical examination in the routine situation. In actual practice, the diagnosis commonly remains unresolved until after surgical excision and histopathologic examination of the specimen. Both of these investigations, however, can be extremely useful to prepare the patient and the treating physician for a major surgical resection if the tumor is clinically suspicious for malignancy.
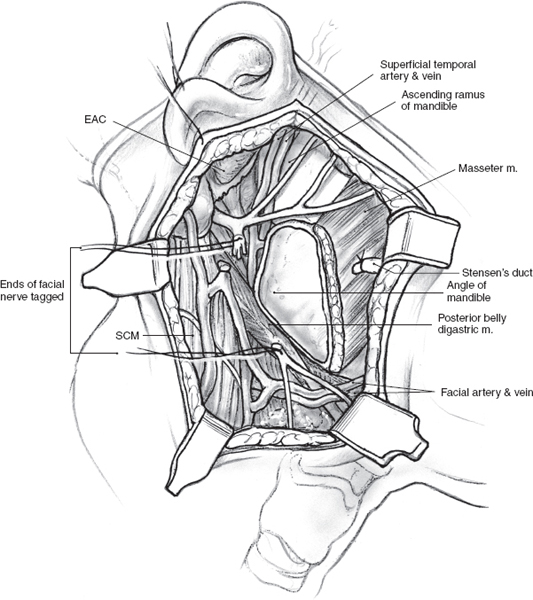
FIGURE 15-21 The surgical field after resection of the tumor shows the divided ends of the peripheral branches of the lower division of the facial nerve. EAC, external auditory canal; SCM, sternocleidomastoid muscle.
Safe and successful surgery of the submandibular gland, like that of the parotid, requires a detailed understanding of regional anatomy (see Chapter 1, Figs. 1–10, 1–12). The intimate relationship of the gland and Wharton’s duct to sensory and motor nerves places the patient at risk for considerable functional deficits if these structures are injured during the operation.
Excision of the Submandibular Gland for a Benign Tumor
The typical patient with a submandibular tumor presents with a diffuse, palpable mass in the submandibular triangle. The skin incision for submandibular gland excision is designed to provide access to the gland with minimal risk to the marginal mandibular branch of the facial nerve (Fig. 15–23). The incision is usually placed along an upper neck skin crease located at least two fingerbreadths from the angle and inferior border of the mandible. The marginal mandibular nerve exits the lower half of the parotid gland and quite frequently loops over the surface of the submandibular gland before turning upward. The surface marking of the nerve usually changes once the patient is on the operating table with the neck extended. It is important that the incision be marked with the patient’s head in flexion so that the appropriate skin crease is chosen. Another useful maneuver is to palpate the pulsations of the facial artery at the inferior border of the mandible. This marks the anterior border of the masseter muscle and the skin incision must be at least two fingerbreadths inferior to this point.
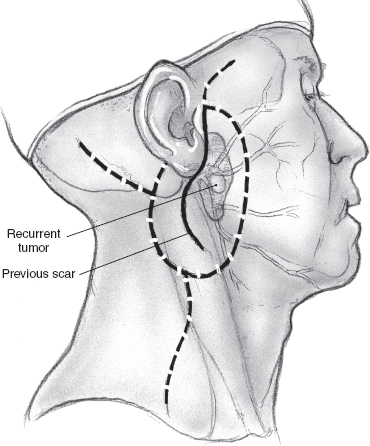
FIGURE 15-22 The skin incision is designed to preserve the vascular supply of the external ear.
The operation is best performed under general anesthesia with an oral endotracheal tube in place. With the patient in the supine position and the neck extended to the opposite side, the skin is incised up to the platysma muscle. Upper and lower skin flaps are elevated in the subplatysmal plane, but the upper flap is not dissected fully until the marginal mandibular nerve has been identified. The upper skin flap is elevated superficial to the platysma muscle for a short distance. The muscle is then incised at the projected location of the marginal nerve, approximately two fingerbreadths anterior and two fingerbreadths below the angle of the mandible. The incision is carefully taken through the platysma muscle down to the underlying fascia over the submandibular gland. A curved hemostat is now used to dissect the platysma off the submandibular fascia, and the muscle is divided carefully over the hemostat to protect the marginal nerve that is usually located on the fascia. Once the nerve has been identified, the remainder of the platysma is divided, and the upper skin flap is elevated to the level of the inferior border of the mandible (Fig. 15–24). The marginal nerve is usually tethered down by the cervical branch of the facial nerve and the posterior facial vein, and complete elevation of the upper flap requires their division. The next step of the operation is to obtain anterior access to the submandibular gland. The anterior belly of the digastric muscle is dissected to allow caudad retraction of the gland (Fig. 15–25). A number of small blood vessels are encountered during this dissection, and these should be ligated and divided to avoid unnecessary blood loss. Further dissection exposes the mylohyoid muscle, and its nerve and blood supply are divided. Dissection continues along the superior border of the gland, between it and the inferior edge of the mandible, to ligate and divide the facial vessels as they exit at the posterior border of the gland. Great care must be taken to avoid injury to the marginal mandibular or other branches of the facial nerve during this dissection. Division of the facial vessels releases the gland and permits caudal retraction to provide exposure of the floor of the submandibular triangle. The free border of the mylohyoid muscle is retracted anteriorly to further open up this space; this brings into view the lingual nerve and its parasympathetic contribution to the submandibular gland. The nerves of the floor of the submandibular triangle are covered by a well-defined layer of fascia and are safe from injury if the surgeon restricts all dissection superficial to this fascia. Caudad traction on the submandibular gland helps delineate the secretomotor ganglion and fibers connecting the lingual nerve to the gland (Fig. 15–26). These fibers are divided carefully, placing the hemostats close to the gland to avoid picking up a knuckle of the lingual nerve in the process. The Wharton’s duct is now identified anteriorly deep to the mylohyoid muscle as it arises from the portion of the gland deep to the muscle. The extent of dissection of Wharton’s duct depends on the indication for submandibular gland excision. If the gland is being resected for chronic sialadenitis, the duct is dissected as far anteriorly as possible to include its entire length with the specimen. The terminal portion of Wharton’s duct hooks over the lingual nerve close to the floor of the mouth, and the nerve is at risk for injury during this maneuver. Extensive dissection of the duct is not necessary if the gland is being resected for neoplastic disease, and the duct is divided at a suitable point closer to the gland and ligated with 3-0 chromic catgut. The hypoglossal nerve is situated deep to Wharton’s duct at this level. Dissection should be restricted to the plane superficial to the fascia covering the nerve, as troublesome bleeding from the venae comitantes can occur, and attempts at hemostasis can result in injury to the hypoglossal nerve. Finally, the facial artery is ligated and divided at the superior edge of the posterior belly of the digastric muscle to deliver the specimen (Fig. 15–27). Hemostasis is secured, and the incision is closed in layers over a Penrose drain.
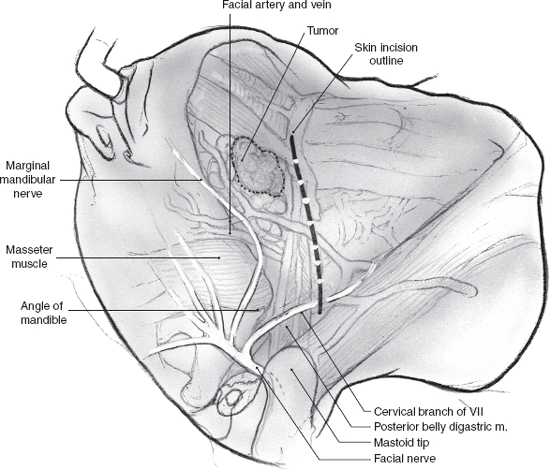
FIGURE 15-23 The typical relationship of a submandibular gland tumor with the mandible and outline of the skin incision.
No special postoperative measures are necessary. The patient is allowed to swallow clear liquids and a pureéed diet for the first 24 hours and should be able to progress to regular diet over the following day. Wound drainage usually tapers off quickly, so that the Penrose drain or suction drain can typically be removed in 1 to 2 days. (A suction drain with absorbable packing over the surgical bed can obviate the need for a dressing and can be removed the following day.)
Radical Excision of the Submandibular Gland for a Malignant Tumor
Surgical excision of the submandibular gland for a suspected malignant tumor with no overt signs of infiltration of adjacent structures is essentially similar to that for benign tumors. In fact, in actual practice the diagnosis of malignancy may not be available to the surgeon until after the gland has been excised and examined by the pathologist. However, if a malignant tumor is suspected, the operation should be modified to include adjacent lymph nodes in the suprahyoid triangle. Small tumors that have not extended beyond the gland can be safely encompassed in such a limited dissection as well. Tumors that have spread beyond the gland to involve adjacent structures need appropriate radical excision depending on the extent of infiltration. A radical resection may include adjacent muscles, nerves including the lingual, hypoglossal, or marginal mandibular, or even a rim or segment of the mandible. A comprehensive neck dissection is indicated for clinically palpable nodes; elective neck dissection is reserved for high-stage tumors.
 Surgery of the Sublingual Glands
Surgery of the Sublingual Glands
Tumors of the sublingual glands are extremely rare, but the frequency of malignancy is high. Due to their location and mode of presentation in the floor of the mouth, it is often difficult to distinguish a sublingual gland tumor from a minor salivary gland tumor of the floor of the mouth. On a practical basis, this hardly makes a difference because the surgical approach to both entities is the same.
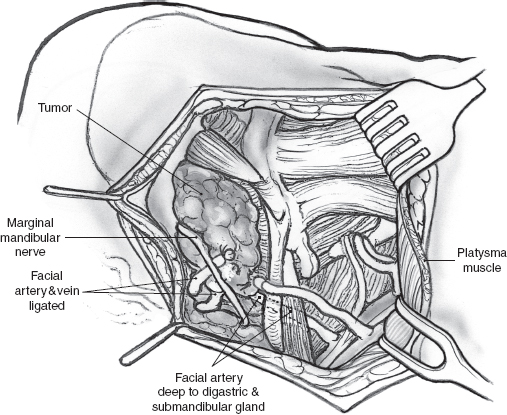
FIGURE 15-24 The platysma is incised, and the marginal mandibular nerve is identified.
Clinical examination, especially bimanual palpation of the floor of the mouth, is a reliable method of assessing local extent of tumor in experienced hands, but radiologic imaging is always helpful. Imaging also enhances clinical examination in evaluation of the relationship of larger tumors to the inner cortex of the mandible and the neck for regional metastases. Biopsy of these submucosal tumors can be difficult if attempted with the usual cup forceps, which may only sample the overlying normal mucosa. A fine dermatologic punch is extremely useful and provides a good tissue core for histologic diagnosis in the office.
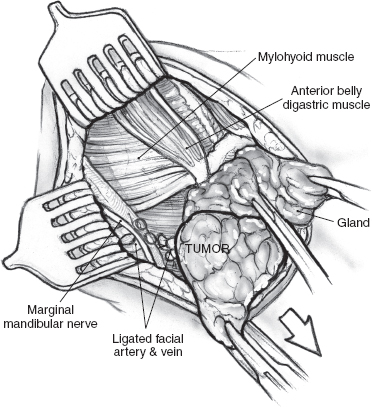
FIGURE 15-25 Dissection of the anterior belly of the digastric muscle in the submental triangle.
Benign tumors or malignant tumors smaller than 2 cm can be readily treated with a peroral local excision. Larger malignant tumors require more radical excision. Accurate preoperative delineation of the extent of the tumor is crucial because any underestimation of involvement of the floor of the mouth musculature or adjacent mandible can lead to a drastic change in the surgical plan. The patient depicted in Fig. 15–28 has a tumor of the sublingual gland that is suitable for peroral local excision. The operation is best carried out under general anesthetic with a nasal endotracheal tube. The jaws are held open with a self-retaining retractor, and an Adair clamp placed on the dorsum of the tip of the tongue helps expose the anterior floor of the mouth. The extent of mucosal resection around the tumor is marked with an electrocautery (Fig. 15–29). Mucosal margins of approximately 1.0 to 1.5 cm around the clinical extent of the tumor should be sufficient. Although the chances of local recurrence are higher with close or positive margins, unnecessary excision of the musculature of the tongue or floor of the mouth can impact the function of the patient. Satisfactory surgical excision with good margins around the tumor in all dimensions, especially the deep soft tissue, therefore depends on the surgeon’s clinical judgment. Because the major sublingual glands lie in close proximity to each other, both glands almost always need to be excised even for unilateral pathology. The sublingual tumor is thus resected with a cuff of the underlying musculature of the ventral tongue for an adequate deep margin coupled with the opposite gland (Fig. 15–30).
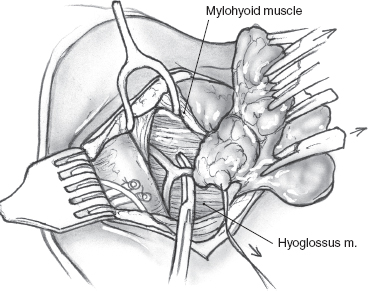
FIGURE 15-26 Division of the secretomotor fibers to the submandibular gland.
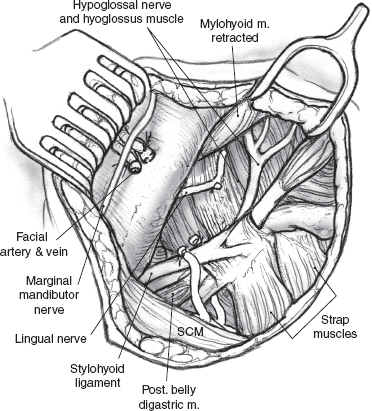
FIGURE 15-27 Anatomical relations after removal of the submandibular gland. SCM, sternocleidomastoid muscle.
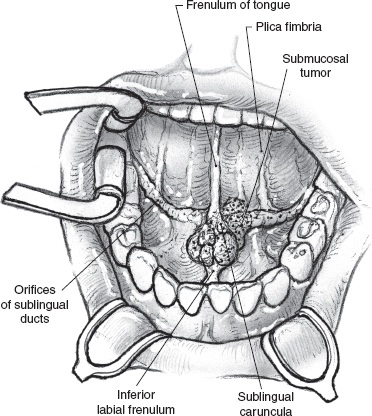
FIGURE 15-28 Surgical exposure through the open mouth is adequate for excision of a small sublingual gland tumor.
The sublingual glands drain directly into the overlying mucosa of the floor of the mouth but are intimately related to Wharton’s ducts and their mucosal puncta. The Wharton’s ducts are identified and can be preserved for transposition if the resection is being performed for a benign tumor. It may be possible to transpose the opposite Wharton’s duct following excision of even a malignant sublingual gland tumor, and every attempt should be made to preserve this function. The ducts are dissected for an appropriate length and transected at an oblique angle with a sharp knife well clear of the tumor. The mucosa of the lateral floor of the mouth at the posterior edge of the surgical defect is buttonholed, and the transected duct is brought out to the surface and sutured in place with fine, absorbable sutures (Fig. 15–31). It is generally advisable to resect the submandibular salivary gland if sufficient length of its duct is not available for it to be transposed or if the tumor is in close proximity to it. The surgical defect is reconstructed using a full-thickness skin graft harvested from the supraclavicular region. The graft is tacked into position using chromic catgut sutures and is secured in place with a Xeroform gauze bolster (Fig. 15–32).
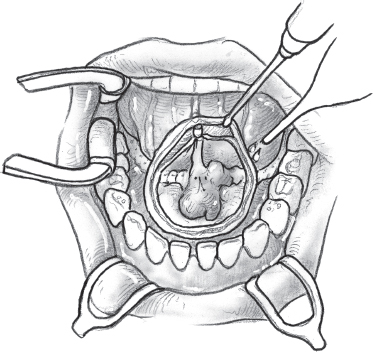
FIGURE 15-29 The extent of mucosal resection around the tumor is marked with an electrocautery.
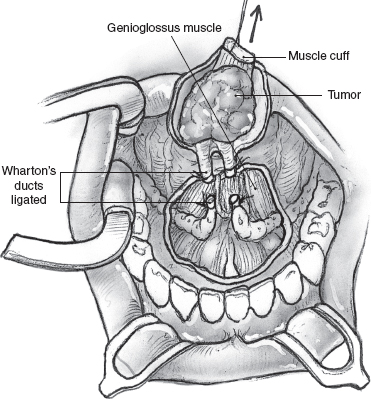
FIGURE 15-30 The sublingual gland tumor is resected with a cuff of underlying muscle for an adequate deep margin of resection.
Postoperatively, the patient is fed through a nasogastric feeding tube for approximately 1 week. Oral hygiene is maintained with frequent oral irrigation and rinsing. The Xeroform bolster is removed at the end of the week, and any skin tags around the surgical defect are trimmed. The nasogastric tube is removed, and the patient is started on clear liquids and gradually progressed to pureéed food. The operation results in no functional deficit, as the mobility of the tongue with limited resections essentially returns to normal.
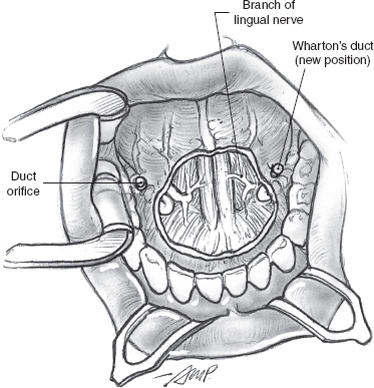
FIGURE 15-31 The stumps of the Wharton’s ducts are transposed posterior to the surgical defect.
Larger tumors with more extensive involvement of the floor of the mouth require radical surgical excision. The tumor is resected en bloc with the structures of the adjacent floor of the mouth. Depending on the extent of the tumor, resection of the lingual nerve, submandibular gland, and even the mandible or the hypoglossal nerve may become necessary. Marginal mandibulectomy is appropriate for a tumor abutting the inner cortex, but actual involvement of the bone is best treated with a segmental resection. Reconstruction of these defects is complicated and may require microvascular free flaps, including the free radial forearm or fibula flaps.
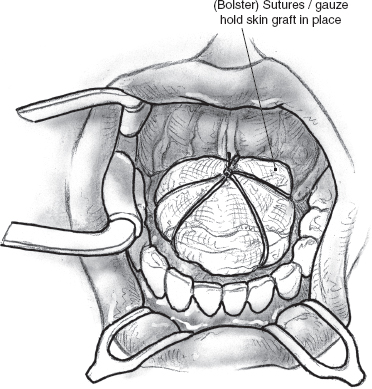
FIGURE 15-32 A Xeroform gauze bolster and black silk tieover sutures are used to secure the skin graft.
 Surgery for Tumors of the Minor Salivary Glands
Surgery for Tumors of the Minor Salivary Glands
The mucosa of the upper aerodigestive tract contains minor salivary glands in varying concentrations. Although tumors of the minor salivary glands are rare, the majority of them are malignant. They are usually submucosal and are covered by a normal-appearing smooth mucosa. Biopsy of these lesions using conventional cup forceps may not sample the submucosal tumor, and as described above, the fine dermatologic punch is capable of producing very reliable tissue cores. Accurate assessment of the local extent of these lesions is crucial to planning the surgical excision and almost always requires radiologic imaging. For minor salivary gland neoplasms involving the nasal cavity or paranasal sinuses, imaging will help not only to differentiate tumor from inflammatory process but also to determine the extent of the neoplasm. Imaging, as well as endoscopy, is necessary for pharyngo-laryngo-tracheal neoplasms.
Imaging studies will also allow differentiation of a parapharyngeal space tumor extending from the parotid and a parapharyngeal tumor of minor salivary gland origin. Parapharyngeal tumor excision via a transcervical approach has been described earlier for parapharyngeal tumors originating from a parotid tumor. Deep lobe parotid tumors with parapharyngeal extension generally require superficial parotidectomy prior to removal of the tumor. Parapharyngeal minor salivary gland tumors may require only dissection of the inferior division of the facial nerve.
Depending on the site of origin of the tumor and its extent, surgical operations for treating minor salivary gland tumors in other locations can range from a simple peroral excision of a hard palate tumor to more complex procedures, such as radical maxillectomy and tracheal sleeve resection. A discussion of all of these procedures is beyond the scope of this chapter, and detailed descriptions are available elsewhere.1
 Conclusion
Conclusion
Surgery for tumors of the salivary glands encompasses a wide range of techniques and procedures. Successful outcome and optimal postoperative rehabilitation depend on a detailed knowledge of regional anatomy, meticulous technique, and multidisciplinary cooperation.
REFERENCES
1. Shah, JP Patel, SG. Head and Neck Surgery and Oncology, 3rd ed. London: Elsevier Science; 2003
2. Spiro, R. Diagnosis and pitfalls in the treatment of parotid tumors Semin Surg Oncol 1991; 7: 20–24
3. Martin, HE Ellis, EB. Biopsy by needle puncture and aspiration. Ann Surg 1930; 92: 169–181
4. Eneroth, CM Zajicek, J Franzen, S. Cytologic diagnosis on aspirates from 1000 salivary gland tumors. Acta Cytol 1966; 10: 440–454
5. Boccato, P Altavilla, G Blandamura, S. Fine needle aspiration biopsy of salivary gland lesions: a reappraisal of pitfalls and problems. Acta Cytol 1998; 42: 888–898
6. Mukunyadzi, P. Review of fine-needle aspiration cytology of salivary gland neoplasms, with emphasis on differential diagnosis. Am J Clin Pathol 2002; 118: S100–S115
7. Heller, KS Dubner, S Chess, Q Attie, JN. Value of the fine needle aspiration biopsy of salivary gland masses in clinical decisionmaking. Am J Surg 1992; 164: 667–670
8. O’Brien, CJ. Current management of benign parotid tumors—the role of limited superficial parotidectomy. Head Neck. 2003; 25 (11): 946–952
9. Witt, RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope 2002; 112 (12): 2141–2154
10. Witt, RL. Facial nerve monitoring in parotid surgery: the standard of care? Otolaryngol Head Neck Surg 1998; 119: 468–470
11. Terrell, J Kileny, P Yian, C, et al. Clinical outcome of continuous facial nerve monitoring during primary parotidectomy. Arch Otolaryngol Head Neck Surg 1997; 123: 1081–1087
12. Wolf, S Schneider, B Suchy, B Eichhorn, B. [Intraoperative facial nerve monitoring in parotid surgery]. HNO 1995; 43: 294–298
13. Brennan, J Moore, E Shuler, K. Prospective analysis of the efficacy of intra-operative nerve monitoring during thyroidectomy, parathyroidectomy and parotidectomy. Otolaryngol Head Neck Surg 2001; 124: 537–541
14. Markkanen-Leppanen, M Pitkaranta, A. Parotidectomy using the haromoic scalpel. Laryngoscope 2004; 114: 381–382
15. Ussmueller, JO Jaehne, M Neumann, BG. The use of diathermy scissors in parotid gland surgery. Arch Otolaryngol Head Neck Surg 2004; 130: 187–189
16. Patel, N Har-El, G Rosenfeld, R. Quality of life after great auricular nerve sacrifice during parotidectomy. Arch Otolaryngol Head Neck Surg 2001; 127 (7): 884–888
17. Conley, J. Search for and identification of the facial nerve. Laryngoscope 1978; 88: 172–175
18. Ru, JA Van Bethan, PP Bleys, RL Lubsen, H Hordijk, G. Landmarks for parotid surgery. J Laryngol Otol 2001; 115: 122–125
19. Byars, LT. Preservation of the facial nerve in operations for benign conditions of the parotid area. Ann Surg 1952; 136: 412–421
20. Kawakami, S Tsukada, S Taniguchi, W. The superficial temporal and retromandibular veins as guides to expose the facial nerve branches. Ann Plast Surg 1994; 32: 295–299
21. Hanazawa, H Miyazaki, S Nishihira, S, et al. Notes on the management of the facial nerve in parotid surgery. Facial Nerve Res Jpn 1988; 8: 203–206
22. Carlson, GW. The salivary glands: embryology, anatomy and surgical applications. Surg Clin North Am 2000; 80: 261–273
23. Vargas, H Galati, L Parnes, S. A pilot study evaluating the treatment of postparotidectomy sialoceles with botulinum toxin type A. Arch Otolaryngol Head Neck Surg 2000; 126: 421–424
24. Sinha, U Ng, M. Surgery of the salivary glands. In: Salivary Gland Diseases. Philadelphia: WB Saunders; 1999: 887–906
25. Kim, S Mathog, R. Platsma muscle-cervical fascia-sternocleidomastoid muscle (PCS) flap for parotidectomy. Head Neck 1999; 21: 428–433
26. Chow, T Lam, C Chiu, P Lim, B Kwok, S. Sternomastoid-muscle transposition improves the cosmetic outcome of superficial parotidectomy. Br J Plast Surg 2001; 54: 409–411
27. Fee, W Tran, L. Functional outcome after total parotidectomy reconstruction. Laryngoscope 2004; 114: 223–226
28. Frey, L. [The auriculotemporal nerve syndrome]. Rev Neurol 1923; 1: 97–104
29. Spiro, RH Martin, H. Gustatory sweating following parotid surgery and radical neck dissection. Ann Surg 1967; 165: 118–127
30. Witt, R. Facial nerve function after partial superficial parotidecotomy: an 11-year review (1987–1997). Otolaryngol Head Neck Surg 1999; 121: 210–213
31. Linder, TE Huber, A Schmid, S. Frey’s syndrome after parotidectomy: a retrospective and prospective analysis. Laryngoscope 1997; 107: 1496–1501
32. Isogai, N Kamiishi, H. Application of medical thermography to the diagnosis of Frey’s syndrome. Head Neck 1997; 19: 143–147
33. Wallis, KA Gibson, T. Gustatory sweating following parotidectomy: correction by a fascia lata graft. Br J Plast Surg 1978; 31: 68–71
34. Kornblut, AD Westphal, P Miehlke, A. A reevaluation of the Frey’s syndrome following parotid surgery. Arch Otolaryngol 1977; 103: 258–261
35. Asal, K Koybasioglu, A Inal, E, et al. Sternocleidomastoid muscle flap reconstruction during parotidectomy to prevent Frey’s syndrome and facial contour deformity. Ear Nose Throat J 2005; 84 (3): 173–176
36. Arad-Cohen, A Blitzer, A. Botulinum toxin treatment for symptomatic Frey’s syndrome. Otolaryngol Head Neck Surg 2000; 122: 237–240
37. Spiro, RH Gerold, FP Shah, JP Sessions, RB Strong, EW. Mandibulotomy approach to oropharyngeal tumors. Am J Surg 1985; 150 (4): 466–469
38. Janecka, IP Tiedemann, K. Skull Base Surgery: Anatomy, Biology, and Technology. Philadelphia: Lippincott Williams & Wilkins; 1996
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


