The management of orbital injuries is one of the most interesting and difficult areas in facial trauma. The consequences of an orbital injury are dramatic. They vary from a loss of vision to diplopia, loss of an eye, epiphora, a disturbing loss of facial sensation, and an unsightly and unacceptable appearance of the eye and the hard and soft tissues around it ( Fig. 10-1 ). These injuries demand a careful attention to detail, but the difficulties are often underestimated and therefore undertreated.
When we look at each other, all areas of the face are important, but there is no doubt that the eyes and how they appear play a very important part in how we initially perceive each other. The eyes are the outward expression of our minds ( Fig. 10-2 ).
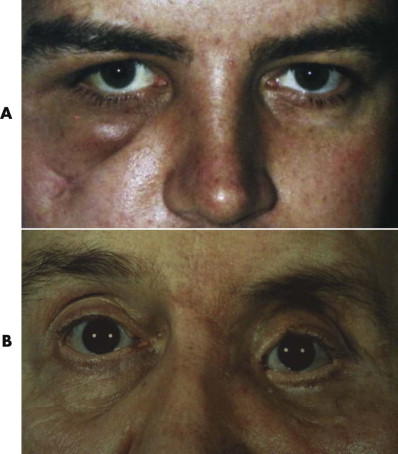
Persistent enophthalmos (sunken eye), hypoglobus or hyperglobus (dropped or raised level of the globe, respectively), strabismus (squint), diplopia (double vision), deteriorated visual acuity or blindness, a false eye, ectropion (eyelid turned out), entropion (eyelid turned in), scarring, fat atrophy, zygomatic malalignment, and canthal dystopia are all significant and debilitating problems ( Fig. 10-3 ). They are common complications of orbital and zygomatic surgery. All lead to distress and to a loss of function (vision) or esthetics or both. Psychological support and counseling may be required.
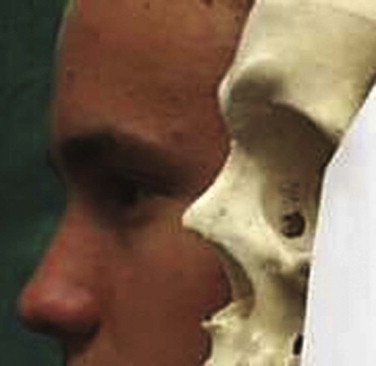
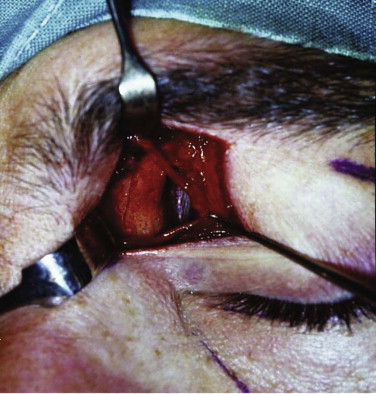
It is essential to take both the soft and hard tissues into consideration. If either is ignored, patient care will be compromised. Treatment must avoid exacerbation of the problem by the poor positioning of facial access incisions ( Figs. 10-4 through 10-6 ). It is possible to expose most of the face and orbit with esthetic incisions: coronal, transconjunctival (sometimes with lateral extension), transcaruncular, lower lid, and intraoral. Endoscopic assessment of injuries and orbital fracture repair is becoming more common.
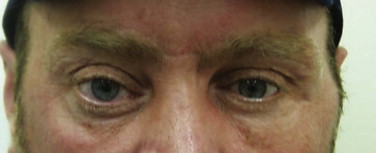
How do we ensure that we get the best results for our patients? The controversies surrounding orbital injuries include the following:
- •
The type, detail, and extent of preoperative clinical examination and investigations that are required
- •
The timing of primary surgery (early or late)
- •
Incisions for fracture exposure
- •
The type of fixation at primary and secondary surgery (i.e., none, multiple, resorbable plates, or non-resorbable plates)
- •
Role of intraoperative guidance (navigation systems)
- •
Bone grafting versus alloplastic materials
- •
The management and prevention of diplopia
- •
The prevention of enophthalmos
- •
The management of infraorbital nerve numbness or dysesthesia
- •
The management of decreasing visual acuity or an acutely blind eye
- •
The management of ocular injuries
- •
Length of follow-up and use of postoperative imaging (computed tomographic [CT] scans)
Assessment, preoperative investigations, collaboration with others, timing of surgery, and methods of treatment, follow-up, and secondary management all demand consideration.
Orbital injuries can occur alone, but most often they are associated with other injuries, such as zygomatic (malar), frontal sinus, naso-orbitoethmoidal, or Le Fort II/III fractures and skull fractures. They are a mixture of low-force injuries (zygomatic fracture, orbital floor, naso-orbitoethmoidal) and high-force injuries (supraorbital rim, orbital roof, frontal sinus) ( Fig. 10-7 ). Orbital injuries are complicated by their proximity to the brain, eye, nasolacrimal apparatus, facial nerve, and sinuses.
The etiology of orbital injuries varies by geographic location. In the United Kingdom, orbital injuries are most often caused by assaults, usually in association with alcohol, but they also occur as a result of road traffic accidents, horse riding accidents, falls, sports, and industrial accidents. Gunshot injuries are rare but are becoming more common. The greater the force, the more comminuted and displaced the fracture and the greater the association with other serious injuries. High-force injuries (e.g., orbital roof, supraorbital rim) have a mortality rate of 12% from associated injuries of the head, neck, and chest. Zygomatic and orbital injuries involving the floor and medial wall are low-impact (low-force) injuries. Injuries involving the medial canthal area (naso-orbitoethmoidal) are associated with intermediate force.
The principles of acute trauma life support for maxillofacial surgery must not be forgotten and are similar to those for any injury ( Fig. 10-8 ):
-
A irway
-
B reathing
-
C irculation, c ervical spine
-
D isability, d rainage (intracranial hematoma, retrobulbar hemorrhage), d rugs (for cerebrospinal leak, fractures)
-
E xpose and e xamine e yes, e ars, and the back of the head
-
F acial nerve
-
G o over it all again
-
H elp
Facial injuries are sometimes considered to be less important than other injuries in the severely injured patient. After any life-threatening injuries have been dealt with, facial injuries should be treated in conjunction with any other procedures (e.g., orthopedic, surgical) rather than left unattended. If a CT scan is requested to rule out an intracranial injury, it is sensible to use that opportunity to assess the orbit and the naso-orbital area, if injury is suspected. Facial lacerations demand attention to detail, and a maxillofacial surgeon should be part of the acute trauma team. Once the patient is stable, lacerations should be explored and sutured within the first few hours.
Blindness is a serious complication of orbital injury. In a prospective study undertaken by al-Qurainy et al., the incidence of severe ocular injury in 438 zygomatic fractures was 12%, with 2.5% of the patients having a significant traumatic optic neuropathy. The incidence of diplopia was 20%, and even with early surgery, long-term diplopia occurred in 1% of the patients. These prospective studies led to the development of a scoring system for eye injuries and highlighted the acronym “BAD ACT”: b lowout fracture, a cuity, d iplopia, a mnesia, and c omminuted t rauma ( Appendix 1 ). If a serious eye injury is suspected or identified, urgent collaboration with an ophthalmologist is required, and consideration of urgent exploration is essential.
Visual acuity must be assessed and recorded. If the patient is conscious but has swelling around the eyes, the assessment can be made easier by explaining to the patient that you need to examine his eyes and that you will be helping him to open his eyes and then asking him to open his eyes while you gently elevate the upper lid and depress the lower lid. If an attempt is made to pry open the eyes, the orbicularis oculi will spontaneously contract and resist the attempt.
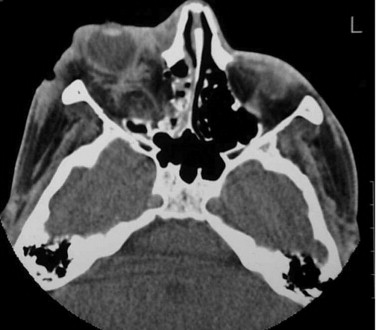
Edema of the retina (Berlin’s edema) is the most common cause of decreased visual acuity, but an intraocular injury, retrobulbar hemorrhage, and optic nerve lesion must be ruled out. Among patients with head and neck trauma, optic nerve injury is the third most common major cranial nerve injury after olfactory and facial nerve injuries. If severe facial injuries are present, these potentially serious problems must be identified during the secondary survey. Traumatic optic nerve lesions (TONL) often occur acutely and are missed or ignored because of other, potentially more serious injuries. Approximately 2% (0.7%-5.0%) of all closed-head injuries and 20% of all frontobasal injuries show some kind of visual pathway damage. In these injuries, it is usually the intracanalicular part of the optic nerve that is affected ( Figs. 10-9 and 10-10 ); and TONLs most commonly are found with frontal (72%) or frontotemporal (12%) injuries.

The first orbital surgery should give the best outcome, and it needs to be thorough. The main aim of orbital surgery is restoration of the anatomy to its normal and esthetic form with preservation of vision, movement, globe position, esthetics, and lacrimation. It is essential to address the whole problem. It is wise to remember the morbidity of surgery, as well as its advantages, but also to consider the problems of neglected treatment. A patient who survives is likely to do better from all aspects if he or she is not left with severe deformities. If there are significant soft tissue injuries, it often is not possible to return the face to its pre-injury state.
Assessment and investigations should be thorough, including access to plain radiography, CT, magnetic resonance imaging (MRI), ultrasonography, and spiral three-dimensional (3-D) reconstruction. Usually, more than one type of investigation is required to get a clear picture. A provisional surgical plan is made, but the final assessment can be made only after open surgical exposure and exploration of the injuries.
Orbital surgery requires a multidisciplinary team including a maxillofacial surgeon, a surgeon with facial plastic surgical experience, an ophthalmic surgeon with access to orthoptists, a neurosurgeon, and a radiologist. Intraoperative fluoroscopy, cameras, and CT screening are becoming more common and are likely to influence the direction of future treatment in this area. Radiological screening helps assess the position of the zygoma and its arch, the correction of enophthalmos, orbital wall and optic canal exploration, and extensive orbital reconstruction. The zygomatic arch, infraorbital rim, and buttress positions are of paramount importance in supporting the zygoma and ensuring optimal orbital reconstruction. Endoscopic assessment of the arch, rim, and orbital floor at the time of surgery is relatively straightforward and is likely to influence future treatment.
Secondary problems are usually related to one or more of the following:
- •
The complexity of the initial soft and hard tissue injury
- •
Delayed treatment
- •
Excessive scarring, including within the orbital fat
- •
Failure of adequate support of soft tissues
- •
Fat atrophy
- •
Inadequate exposure, reduction or fixation of orbital/ zygomatic fractures
- •
Comminuted zygomatic fractures ( Fig. 10-11 ), missed medial and floor injuries

FIGURE 10-11 Inadequate fixation leading to flat left zygoma with lateral canthal drift. - •
Bone loss
Clinical Assessment of Orbital Injury
A thorough history and clinical examination are undertaken ( Appendix 2 ) to determine the possibility of a penetrating, chemical, or blunt injury to the eye or soft tissues. Evidence of a head injury (amnesia), a neck injury (10% of cases), loss of smell (anosmia), deterioration or loss of vision, development of double vision (diplopia—vertical, horizontal, or rotatory), eye movements (painful or not), and eye position in all three planes (proptosis or enophthalmos, hypoglobus or hyperglobus, or an orbital dystopia) should be recorded.
Orbital trauma can result in displacement of the globe in any direction. Proptosis occurs in the acute phase due to hematoma or swelling of orbital tissues, or both. As the swelling subsides, proptosis resolves and may become enophthalmos. Persistent proptosis may be caused by subperiosteal hematoma or intraorbital bone fragments. Enophthalmos is a common late sequela and may result from a combination of expansion of the orbit, prolapse of soft tissue through a blowout fracture, necrosis of soft tissue (rare), and intraorbital fibrosis. There is often an associated impairment of ocular motility. Vertical displacement of the globe is common. Upward displacement may result from hematoma or edema in the acute phase or from orbital floor impaction causing a reduction in orbital volume. Downward displacement (hypoglobus) is far more common, particularly in patients with comminuted orbital floor or lateral wall fractures or roof fractures. Horizontal lateral displacement of the globe can result particularly from naso-orbitoethmoidal fractures and from severing of the medial canthal ligament. Traumatic herniation of the globe into the maxillary sinus can occur. Good recovery of visual function and esthetics is achievable after effective repair.
Eye position can be measured with the use of an exophthalmometer but is best assessed from CT or MRI scans. The exophthalmometer is of little benefit for patients with lateral orbital disruption, the most common orbital injury. Surgeons have developed exophthalmometers based on the ear canals and supraorbital positions, but these are less accurate than CT scan assessment, which also allows an assessment of orbital fat volume and helps determine the need for surgery.
Facial sensation is tested. Altered facial sensation around the orbit may be the only initial clinical sign of a blowout fracture. The alar of the nose should be gently touched, and the patient should be asked if she can feel one side and then the other and whether they feel the same. Swelling can give a false impression of true nerve damage.
Facial nerve injury compounds any orbital injury and should be actively sought. If the nerve is severed through a laceration, the laceration should be explored and the nerve primarily repaired with the aid of a microscope, unless it is a peripheral injury.
Intercanthal (medial and lateral) and interpupillary measurements are taken (see Appendix 2 ). The use of a local anesthetic to enable a full clinical assessment for medial canthal disruption is advised.
Mouth opening should be assessed, because any displacement of the zygomatic complex can impinge on the temporalis muscle or coronoid process and interfere with jaw movement. Trismus is not an uncommon complication of a zygoma or orbital fracture.
It is an advantage to have a diagram or photograph in the notes to demonstrate grazes, bruises, and the presence of periorbital or lid lacerations and to record any loss of soft or hard tissue.
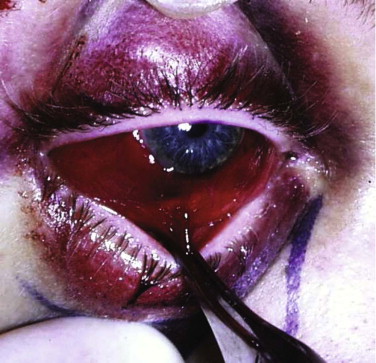
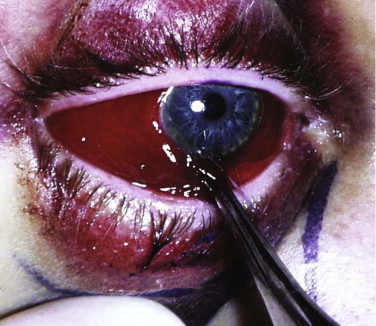
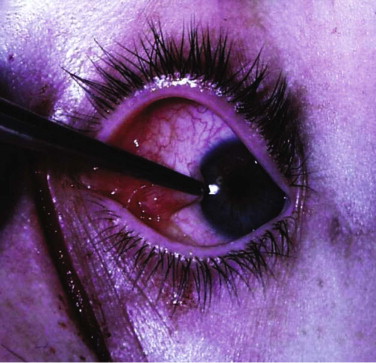
Forced duction tests under local anesthesia can be helpful but are not necessary in the conscious patient ( Figs. 10-12 through 10-14 ). The clinical sign of pain on looking up or laterally with diplopia is evidence of entrapment. The pain is thought to be caused by disturbance of the periosteum by the entrapped tissue. A Hess test helps display any restriction, but it is important to look for the clinical sign of pain (i.e., slight grimace) and retraction of the globe on eye movement.
Applied Surgical Anatomy
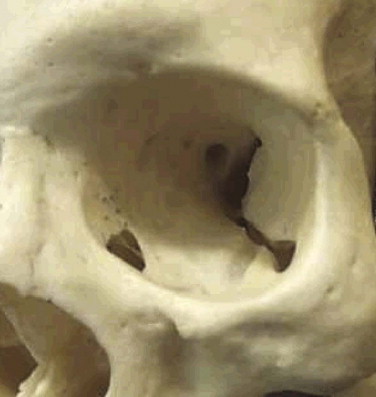
The orbit ( Figs. 10-15 through 10-17 ) has several important anatomical features. Knowledge and appreciation of the anatomy enables one to understand the injuries and to postulate how to reconstruct them. The orbital skeleton should be returned to its norm with enough strength to resist disrupting forces. There is argument about simple elevation and single, two-point, and three-point fixation approaches. It is recognized that even with frontozygomatic and infraorbital rim fixation, posterolateral orbital rim rotation can be missed. It is advisable with unstable zygoma fractures to view the frontozygomatic fracture and either the buttress (intraoral) or the zygoma/sphenoidal fracture line to prevent the development of enophthalmos.
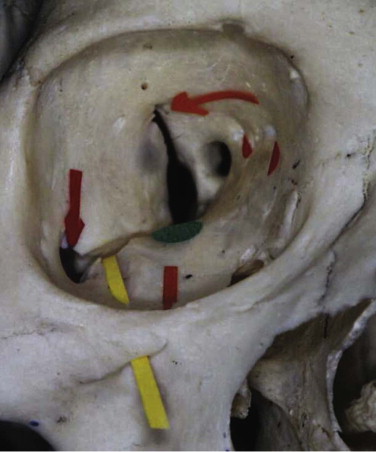
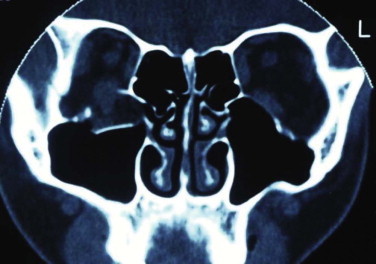
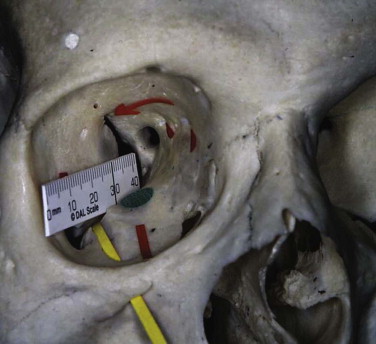
The orbit can be anatomically classified in many ways. It has been described in relation to its bony components; it is made up of seven bones. It has been divided into anterior, middle, and posterior thirds to help differentiate the sites related to hypoglobus (anterior orbital floor), enophthalmos (middle), and visual or neurological problems (posterior) (see Fig. 10-9 ). Correction of hypoglobus requires lifting of the globe and repair (with or without bone grafting) of the anterior half of the orbit. Prevention or correction of enophthalmos requires the globe to be pushed forward with repair of the lateral and medial walls and reconstruction of the retrobulbar bulge.
The orbit has also been described according to its anatomical walls: it has a floor, a medial wall, a roof, a lateral wall, an apex, and an orbital rim. This method is the easiest to comprehend, and combined with the other classifications, it allows for a logical anatomical assessment and a logical treatment of orbital injuries.
The orbit is a quadrangular pyramid with its base on the facial surface. Its apex is the optic foramen and the medial end of the superior orbital fissure.
The orbital rim is composed of cortical bone. Its strength arises from its circumorbital continuity. If the rim is broken, the strength—particularly of the inferior, medial, and lateral walls of the orbit—is significantly decreased. Dissipation of the impact required to fracture the rim often transmits the forces to the floor and medial wall, leading to concomitant damage to the floor and wall, while leaving the rim intact. A patient with an isolated orbital rim fracture may initially have few symptoms, exhibiting only infraorbital anesthesia, but may present later with enophthalmos or dysesthesia, epiphora (secondary to nasolacrimal obstruction), or dacroycystorhinitis. These patients should be monitored for at least 6 weeks before discharge (see Fig. 10-16 ). Symptomatic infraorbital nerve problems warrant treatment.
The floor of the orbit is very thin and S -shaped. It is concave anteriorly and convex posteriorly (see Figs. 10-15 and 10-17 ). It is traversed anteriorly by the infraorbital groove and canal (lateral to medial). The infraorbital canal is suspended from the floor. This groove and canal carry the infraorbital nerve from the pterygopalatine fissure below the inferior orbital fissure to the infraorbital foramen and further weaken the floor. This anatomical relationship accounts for the clinical sign of facial numbness, paresthesia, or dysesthesia affecting the alar of the nose, cheek, upper lip, and anterior teeth after an orbital floor or zygomatic fracture. The floor is fractured either by buckling (dissipation of orbital rim forces) or by rapid expansion of the orbital contents, which leads to a fracture of the floor or the medial orbital wall. Floor fractures are usually medial to the nerve. If a fracture involves the whole floor, the infraorbital nerve supports the floor like a piece of string, camouflaging the initial clinical signs of hypoglobus and enophthalmos.
The anatomy of the postbulbar bulge (maxillary sinus expansion posterior to the globe) is important in maintaining the eye’s anterior-posterior position (assuming that the soft tissue support is damaged) and must be reconstructed to prevent enophthalmos. Volume changes on CT examination illustrate clearly the importance of this area in the development of enophthalmos. This area is often not explored because of a reluctance to dissect far enough into the orbit. It is essential to dissect the inferior fissure free from the orbital contents, at least to behind the infraorbital nerve (see Fig. 10-16 ). The inferior orbital fissure is traversed by only minor vessels, lymphatic channels, and fibrofatty tissue. The infraorbital nerve passes below the fissure to enter the infraorbital groove. These tissues are carefully dissected under magnification by a combination of bipolar diathermy and knife dissection. The floor must be fully explored, and in the adult this usually requires a dissection along the floor and lateral wall for at least 35 mm (see Fig. 10-17 ).
Most failed explorations are caused by a reluctance to adequately free up this area and get completely around any floor fracture or defect (navigation systems may be helpful). The difficult areas are (1) the junction of the infraorbital groove and the inferior fissure and (2) identification of the posterior aspect of the retrobulbar bulge as it passes posteriorly and medially to the inferior fissure. The periosteum needs to be incised on either side of the fissure and elevated gently. Care must be taken at the junction of the infraorbital groove and fissure to dissect the infraorbital nerve free from the orbital tissues. This subperiosteal dissection, using careful eye retraction, magnification, and a light source (head light), enables a full exploration of the orbital floor and its lateral and medial walls. The transconjunctival or lower eyelid skin incision does not need to be extended if the orbital periosteum is well mobilized. The inferior rectus and inferior oblique muscles are protected by remaining in a subperiosteal plane. The anatomy of the nasolacrimal sac and canal should be kept in mind.
It has been suggested that an inferior orbitotomy may help in exploring large blowout fractures. In our experience, an inferior orbitotomy is used occasionally in secondary procedures but usually is not necessary in the acute situation. An inferior orbitotomy may be useful to decompress the infraorbital nerve in patients with infraorbital nerve dysesthesia or persistent numbness ( Figs. 10-18 through 10-22 ). Resolution and protection of cheek and lip sensation is best achieved, even with minimal infraorbital rim disruption, by reduction and fixation of the fractured bone at the frontozygomatic or infraorbital rim or both.
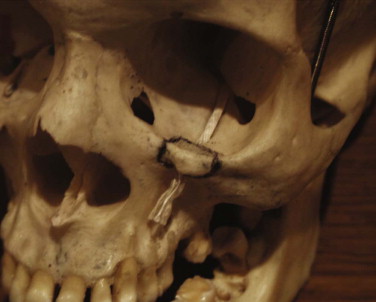
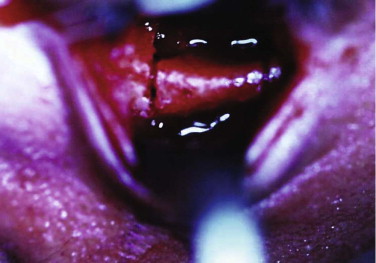
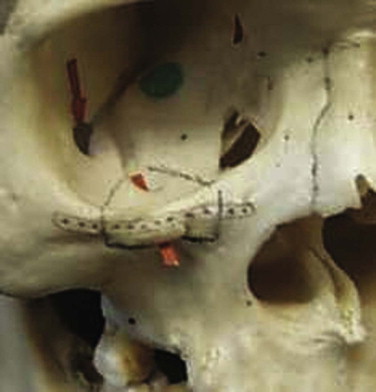
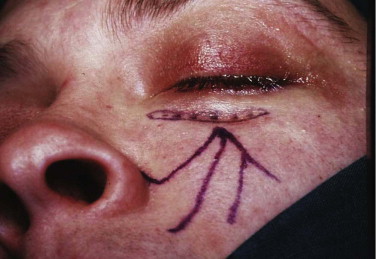
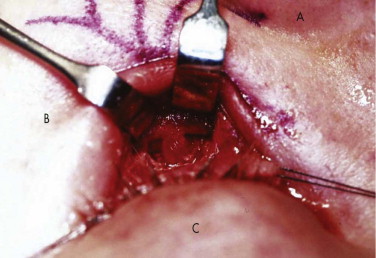
The anterior floor supporting the globe is reconstructed (usually with bone grafting) to prevent hypoglobus. It is our preference to use autogenous bone from the cranium, mandible, or opposite lateral maxillary sinus wall, although nasal septum, ear cartilage, and fascia lata have also been used with success. Alloplastic material (Silastic) has a tendency to extrude, but Silastic remains the most frequently used material in the United Kingdom. Resorbable material, although useful in small defects, potentially leaves the patient with late enophthalmos after resorption. Very large defects are well restored with a combination of metal (titanium or Vitallium) and autogenous bone. The posterior floor (bulbar bulge) is reconstructed with layers of bone to correct and prevent enophthalmos. There is still a reluctance to undertake bone grafting in the acute management of these injuries. Primary bone grafting in facial injuries has been shown to give good long-term results.
The medial wall is paper thin ( Fig. 10-23 ). It obtains its strength from the continuity of the ethmoidal air cells and from their multiple septa. When it is fractured, the forces of injury are rapidly dissipated, thus protecting the brain and eye. Nasal fractures frequently involve the medial wall and the inferior orbital rim.
Although nasal radiographs are not usually taken, there should be a high index of suspicion of medial wall injuries in cases of anterior collapsed nasal fracture. If there is any clinical indication of nasal or cheek numbness, radiographs should be taken. Medial wall defects tend to be small and minimally displaced, and they are easily missed. There is a high incidence (30%-50%) of medial wall fractures associated with floor fractures. Medial wall injuries contribute to enophthalmos and can cause horizontal diplopia. The medial wall is traversed by the anterior and posterior ethmoidal arteries at about 24 and 34 mm, respectively, from the anterior lacrimal crest (orbital rim). If the medial wall is being explored, these arteries should be identified and coagulated. Bleeding can easily be arrested by bipolar diathermy. Although these are relatively low-pressure vessels, they do contribute to the problems of visual impairment, ophthalmoplegia, and proptosis after retrobulbar hemorrhage ( Fig. 10-24 ).
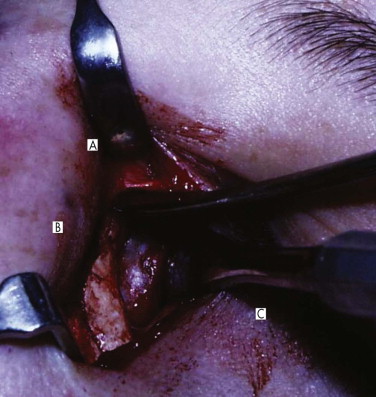
The nasolacrimal sac is sited anteriorly between the orbital rim and the posterior lacrimal crest. It is protected by the anterior and posterior bands of the medial canthal ligament ( Figs. 10-25 and 10-26 ) and drains through the nasolacrimal duct to the inferior meatus of the nose. In the treatment of orbital injuries, it is important to protect the canaliculi, the sac, and the duct when placing incisions and for osteosynthesis. Obstruction in the duct or damage to the sac can cause epiphora and recurrent dacroycystorhinitis.
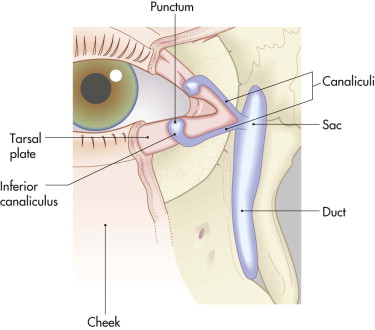
Telecanthus, medial canthal dystopia, nasal deformity, epiphora, and dacroycystitis are also complications of medial orbital wall and nasal injuries; they can be addressed only by considering them in the acute phase and reconstructing the areas anatomically.
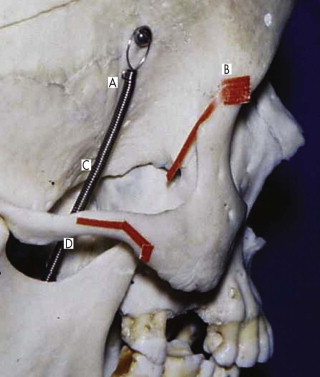
The lateral orbital wall is fairly strong. It is supported by the temporalis muscle but weakened inferiorly by the inferior orbital fissure and posterosuperiorly by the superior orbital fissure. It is sensible to understand the relationship between these two fissures (see Figs. 10-9 and 10-15 ). Fractures in the lateral wall and displacement are usually associated with zygomatic fractures. Inferior displacement of the zygoma leads to displacement of the lateral canthus and to a unilateral mongoloid slant to the palpebral fissure (see Fig. 10-11 ). Undercorrected or overcorrected lateral displacement (lateral wall) is the most common cause of enophthalmos and hypoglobus. If zygomatic fixation at the frontozygomatic suture is not secure, the masseteric and temporalis muscles can pull the zygoma inferiorly, leading to an osteodistraction force at the frontozygomatic suture with elongation of the lateral wall. This is a very important concept in secondary reconstruction and explains the need to resect a measured piece (usually 5 mm) of the lateral orbital rim in a malar (zygomatic) osteotomy ( Fig. 10-27 ).
Adequate reduction of the zygoma is difficult to determine on the table, in particular the final volume of the orbit. As a result supplementary methods like that of ultrasonography, fluoroscopy, CT navigation, or endoscopy have become more popular. CT navigation is currently the “gold standard” to ensure a perfect reduction and correction of the orbital volume. Postoperative radiographs or some accurate analysis are an integral part of assessment of the postoperative positions of the zygoma and orbit. Reoperation should be undertaken earlier rather than later to avoid the long-term sequelae. In some countries (United States, Australia), postoperative spiral CT scans are taken for moderate to complex orbital reconstructions to assess orbital volume, zygoma and bone graft position, and orbital wall reconstruction. This is the gold standard and enables clinicians to improve future treatment for these patients, but of course there are concerns about the radiation given to this sensitive area, without any clear evidence of an audited benefit.
The orbital roof is protected by the very strong supraorbital rim, but fractures of the roof are commonly seen in association with frontal sinus and naso-orbitoethmoidal fractures ( Figs. 10-28 and 10-29 ). These can be blowout fractures but more commonly are blow-in fractures, leading to hypoglobus and proptosis. This injury is also associated with diplopia due to displacement of the trochlea, which transmits the tendon of the superior oblique muscle of the eye. Consideration of the orbital roof is essential in any craniofacial injury. The orbit’s relationship with the frontal sinus is a natural defense mechanism against injury to the brain or eye, because the forces are dissipated through fracturing of the walls of the air-filled sinus. If injured, this area warrants reconstruction, because damage to the frontonasal duct can lead to mucoceles, sinusitis, and possible meningitis. An isolated blow-in fracture with proptosis is relatively easily treated. This is best done in collaboration with a neurosurgeon and by means of a subcranial or transcranial approach.
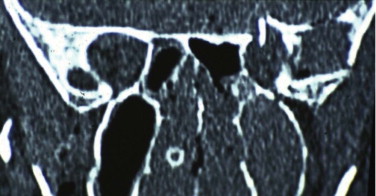
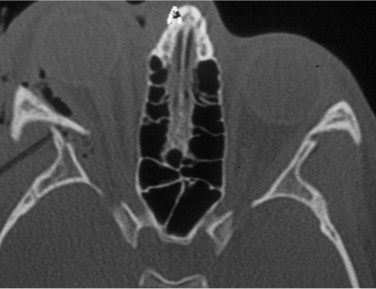
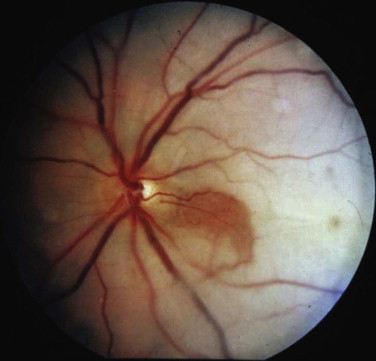
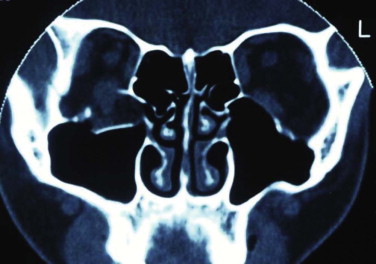
The orbital apex is not well understood (see Figs. 10-9, 10-10, 10-15, and 10-17 ). It transmits the optic nerve with its retinal artery, which is an end artery. Injury or constriction of this vessel may lead to acute or insidious blindness after an orbital injury (i.e., TONL) and development of traumatic optic atrophy ( Fig. 10-30 ). There is an increasing tendency, although unproven need, to explore this area to decompress the optic nerve (see later discussion of ocular injuries).
Other important anatomical factors in the apical area include the posterior aspects of the inferior and superior orbital fissures, the tendinous ring origin of the ocular muscles, and the oculomotor, trochlear, abducens, and ophthalmic nerve branches. Injury to this area leads to a number of well-recognized syndromes. The orbital apex syndrome is blindness (optic nerve injury). The clinical signs of superior orbital fissure syndrome are:
- •
gross and persistent periorbital edema.
- •
proptosis (due to loss of tone of muscles of the eye).
- •
ophthalmoplegia and ptosis (third, fourth, and sixth cranial nerves).
- •
subconjunctival hemorrhage.
- •
pupil dilatation.
- •
an absent direct light reflex but a persistent indirect light reflex (i.e., second nerve is normal).
- •
loss of accommodation, corneal reflex, and sensation to the forehead (frontal nerve).
Treatment for these symptoms has been conservative in the past. It is now advisable to consider orbital apex exploration if the patient has an acute visual loss with no intraocular cause, in an effort to save vision.
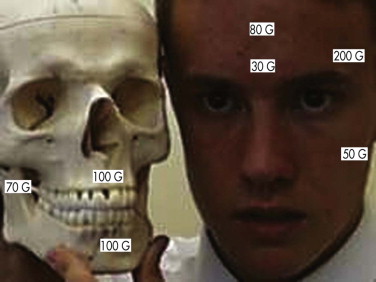
It is necessary when operating to understand the dimensions of the orbit and how far it is safe to dissect down any individual wall. For this reason, the availability of a dried or plastic skull in the operating room is advocated to facilitate understanding of orbital dissection. It is possible through orbital incisions to mobilize the orbital contents so that they are free except for the superior fissure, the tendinous muscle origin and optic nerve, and the nasolacrimal and medial canthal area. This allows for a thorough exploration of all the orbital walls, floor, and roof. It is also a good use of a navigation system to ensure precise access to the damaged area.
In the case of avulsive orbital defects, the remaining orbit should be reconstructed. The bony defect should be reconstructed with primary autogenous bone grafting, supported with rigid fixation (low-profile plates), and covered with soft tissue through local rotation flaps or free vascular tissue, rather than waiting for the scarring process to proceed. Scarring will further compromise the orbital outcome.
Applied Surgical Anatomy
The orbit ( Figs. 10-15 through 10-17 ) has several important anatomical features. Knowledge and appreciation of the anatomy enables one to understand the injuries and to postulate how to reconstruct them. The orbital skeleton should be returned to its norm with enough strength to resist disrupting forces. There is argument about simple elevation and single, two-point, and three-point fixation approaches. It is recognized that even with frontozygomatic and infraorbital rim fixation, posterolateral orbital rim rotation can be missed. It is advisable with unstable zygoma fractures to view the frontozygomatic fracture and either the buttress (intraoral) or the zygoma/sphenoidal fracture line to prevent the development of enophthalmos.
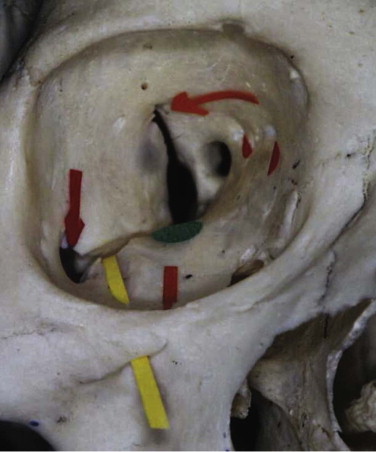
The orbit can be anatomically classified in many ways. It has been described in relation to its bony components; it is made up of seven bones. It has been divided into anterior, middle, and posterior thirds to help differentiate the sites related to hypoglobus (anterior orbital floor), enophthalmos (middle), and visual or neurological problems (posterior) (see Fig. 10-9 ). Correction of hypoglobus requires lifting of the globe and repair (with or without bone grafting) of the anterior half of the orbit. Prevention or correction of enophthalmos requires the globe to be pushed forward with repair of the lateral and medial walls and reconstruction of the retrobulbar bulge.
The orbit has also been described according to its anatomical walls: it has a floor, a medial wall, a roof, a lateral wall, an apex, and an orbital rim. This method is the easiest to comprehend, and combined with the other classifications, it allows for a logical anatomical assessment and a logical treatment of orbital injuries.
The orbit is a quadrangular pyramid with its base on the facial surface. Its apex is the optic foramen and the medial end of the superior orbital fissure.
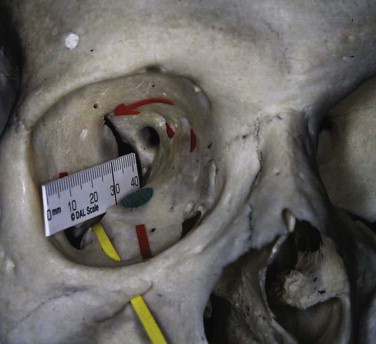
The orbital rim is composed of cortical bone. Its strength arises from its circumorbital continuity. If the rim is broken, the strength—particularly of the inferior, medial, and lateral walls of the orbit—is significantly decreased. Dissipation of the impact required to fracture the rim often transmits the forces to the floor and medial wall, leading to concomitant damage to the floor and wall, while leaving the rim intact. A patient with an isolated orbital rim fracture may initially have few symptoms, exhibiting only infraorbital anesthesia, but may present later with enophthalmos or dysesthesia, epiphora (secondary to nasolacrimal obstruction), or dacroycystorhinitis. These patients should be monitored for at least 6 weeks before discharge (see Fig. 10-16 ). Symptomatic infraorbital nerve problems warrant treatment.
The floor of the orbit is very thin and S -shaped. It is concave anteriorly and convex posteriorly (see Figs. 10-15 and 10-17 ). It is traversed anteriorly by the infraorbital groove and canal (lateral to medial). The infraorbital canal is suspended from the floor. This groove and canal carry the infraorbital nerve from the pterygopalatine fissure below the inferior orbital fissure to the infraorbital foramen and further weaken the floor. This anatomical relationship accounts for the clinical sign of facial numbness, paresthesia, or dysesthesia affecting the alar of the nose, cheek, upper lip, and anterior teeth after an orbital floor or zygomatic fracture. The floor is fractured either by buckling (dissipation of orbital rim forces) or by rapid expansion of the orbital contents, which leads to a fracture of the floor or the medial orbital wall. Floor fractures are usually medial to the nerve. If a fracture involves the whole floor, the infraorbital nerve supports the floor like a piece of string, camouflaging the initial clinical signs of hypoglobus and enophthalmos.
The anatomy of the postbulbar bulge (maxillary sinus expansion posterior to the globe) is important in maintaining the eye’s anterior-posterior position (assuming that the soft tissue support is damaged) and must be reconstructed to prevent enophthalmos. Volume changes on CT examination illustrate clearly the importance of this area in the development of enophthalmos. This area is often not explored because of a reluctance to dissect far enough into the orbit. It is essential to dissect the inferior fissure free from the orbital contents, at least to behind the infraorbital nerve (see Fig. 10-16 ). The inferior orbital fissure is traversed by only minor vessels, lymphatic channels, and fibrofatty tissue. The infraorbital nerve passes below the fissure to enter the infraorbital groove. These tissues are carefully dissected under magnification by a combination of bipolar diathermy and knife dissection. The floor must be fully explored, and in the adult this usually requires a dissection along the floor and lateral wall for at least 35 mm (see Fig. 10-17 ).
Most failed explorations are caused by a reluctance to adequately free up this area and get completely around any floor fracture or defect (navigation systems may be helpful). The difficult areas are (1) the junction of the infraorbital groove and the inferior fissure and (2) identification of the posterior aspect of the retrobulbar bulge as it passes posteriorly and medially to the inferior fissure. The periosteum needs to be incised on either side of the fissure and elevated gently. Care must be taken at the junction of the infraorbital groove and fissure to dissect the infraorbital nerve free from the orbital tissues. This subperiosteal dissection, using careful eye retraction, magnification, and a light source (head light), enables a full exploration of the orbital floor and its lateral and medial walls. The transconjunctival or lower eyelid skin incision does not need to be extended if the orbital periosteum is well mobilized. The inferior rectus and inferior oblique muscles are protected by remaining in a subperiosteal plane. The anatomy of the nasolacrimal sac and canal should be kept in mind.
It has been suggested that an inferior orbitotomy may help in exploring large blowout fractures. In our experience, an inferior orbitotomy is used occasionally in secondary procedures but usually is not necessary in the acute situation. An inferior orbitotomy may be useful to decompress the infraorbital nerve in patients with infraorbital nerve dysesthesia or persistent numbness ( Figs. 10-18 through 10-22
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


