Chapter 9 The tooth-coloured restorative materials III
Glass ionomer cements
Learning Objectives
From this chapter, the reader will:
• Understand what glass ionomer cements are
• Understand the significance of their constituents with regard to their handling and clinical applications
• Understand the properties of this type of materials and their relationship to clinical manipulation and performance
• Understand the indications, contraindications and limitations of these cements
• Know the names of currently available commercial products
• Have an increased appreciation of how to use these materials more effectively.
Introduction
The next group of tooth-coloured restorative materials are the glass ionomer cements. This generic group of materials is distinguished by setting involving an acid–base reaction, requiring the presence of water. All commercially available glass ionomer materials involve a reaction of an acidic liquid with a basic glass. From a chemical and ISO terminological perspective, the term glass polyalkenoate cements is strictly speaking more correct when referring to this group of materials. The original term (glass ionomer cements) excludes some of the acids being used in the currently available products.
History
![]()
The term a light-cured glass ionomer is often used by dental manufacturers in promotional literature. It is important to stress that this refers to materials which are not true glass ionomer cements. Light curing is required because of the addition of a resin to the material (together with the chemicals needed to effect light polymerization of the material). These materials should be referred to as resin-modified glass ionomer cement. Chapter 10 deals with these materials.
Components of a Glass Ionomer
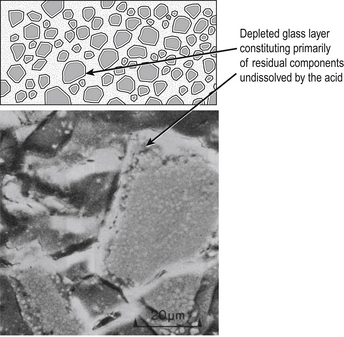
The material is the product of the chemical reaction between the glass and an acid when the two components are mixed together. There is an initial dissolution of the surface of the powder. The soluble components of the glass react with the polyacrylic acid to form a matrix. After setting, the residual unreacted material is encased in this salt matrix, which holds the cement together (Figure 9.1). The set material is therefore a cored structure in which only the surface of the glass has reacted to permit the binding of the glass particles together.
Glass
• Adding additional elements, e.g. strontium and lithium, to impart radiopacity
• Making the cement more translucent by altering the aluminium/silica ratio
• Altering the rate of ion release, an important factor in determining solubility, setting characteristics and release of fluoride.
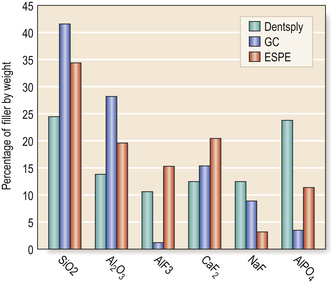
The glass has a formulation based on the firing of a combination of chemicals (Table 9.1). Figure 9.2 shows some typical glass formulations for commonly available cements and highlights the substantial variation in the formulations of glass which may be used to produce a glass ionomer cement.
Table 9.1 Compounds contained in the glass of a typical glass ionomer cement
| Compound | Percentage composition |
|---|---|
| Alumina (aluminium oxide) | 14.2–28.6 |
| Silica (silicon dioxide) | 30.1–41.9 |
| Calcium fluoride | 12.8–34.5 |
| Aluminium fluoride | 1.6–11.0 |
| Aluminium phosphate | 3.8–24.2 |
| Sodium fluoride | 3.6–12.8 |
Manufacturing process for the glass component
The glass mixture is heated to a temperature between 1150°C and 1450°C. The molten mass is then poured onto a metal plate and then into water, a process termed shock cooling. The glass is then broken up to form a glass frit (Figure 9.3). All the glasses for the currently available cements are then either wet or dry milled to produce small particles of glass. Various ranges of particle size are used depending on the requirements and usage of the cement. The larger glass particle sizes are used in those cements intended as restorative materials (up to 20 μm) while the smaller particle sizes (<5 μm) are used for luting cements.
Frit: This is the product of pouring molten glass into water. It usually consists of small particles of glass which will be ground up to smaller size later in the glass preparation process. The size of the glass particles is determined by the use or application.
Wet milled: The glass particles are placed in a cylindrical ceramic vessel with a volume of water and a number of ceramic balls. The whole assembly is then rotated. The ceramic balls tumble around the vessel grinding the glass frit down in size. The longer the tumbling process the finer the particles of glass.
Dry milled: as for wet milling but with the exclusion of the water.
Acid
Homopolymer: a polymer that contains one single monomer type.
Ion: An ion is an atom or molecule where the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge.
Copolymerization: the method of chemically synthesizing a copolymer.
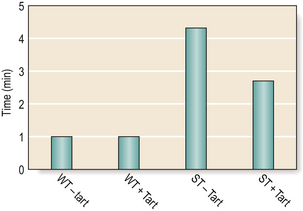
Changes in molecular weight will also influence the working and setting time of the cement. The higher the molecular weight (and so the viscosity), the shorter the working and setting times. A small amount of tartaric acid is added to all materials to accelerate the setting phase of the reaction while maintaining the working time (Figure 9.4).
Presentation
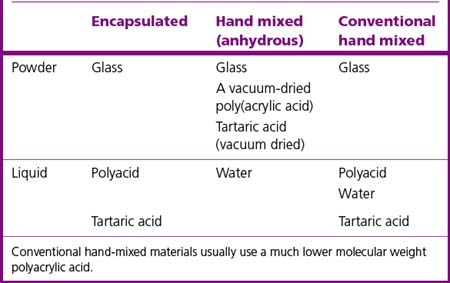
Two typical presentations of glass ionomer cements commonly exist: encapsulated and hand mixed. The chemical constituents of each are described in Table 9.2.
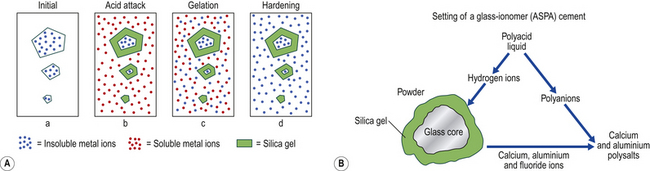
Setting Reaction
The setting reaction of a glass ionomer cement involves many stages (Figure 9.5). On mixing the cement paste:
1. The glass is attacked by hydrogen ions from the acid, liberating aluminium and calcium ions. Fluoride and sodium ions are also released.
2. The pH of the aqueous phase rises and leads to a further ionization of the polyacrylic acid.
3. This leads to migration of aluminium and calcium cations into the aqueous phase.
4. Ionization of the polyacrylic acid leads to unwinding of the polymer chain. This causes the viscosity of the paste to increase, and the cation concentration also increases.
Anion: an ion carrying a negative charge
Cation: an ion carrying a positive charge.
Cross-linking: bonds which link one polymer chain to another.
Polyanion: a molecule carrying a large number of negative charges. In the case of a glass ionomer cement, there may be a number of carboxyl groups on one polymer chain and these will individually react with metal ions which are in solution.
Setting Time
The etching of glass ionomer cements has been recommended in some restorative procedures to enhance the union between the two materials such as in the sandwich or laminate technique, where a resin composite forms the surface of the restoration and the glass ionomer acts as a base. The etching may, however, damage the cement structure. The earlier the etching process is performed in the glass ionomer cement setting reaction, the more likely it is that the salt matrix will be damaged as it has not matured. If etching is carried out within 5 minutes of placement, the matrix is completely removed within 15 seconds of applying the 37% phosphoric acid etchant (Figure 9.6). Successful etching of the glass ionomer cement can only be achieved after the matrix has matured, that is, after 24 hours. After this time the rate of removal of the salt matrix is much slower and a partial surface removal of the salt matrix will occur.
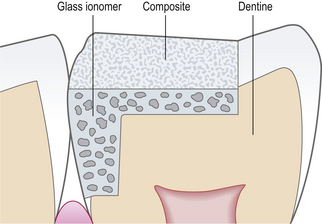
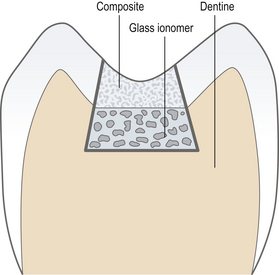
Open sandwich: A restoration in a tooth in which two restorative materials are used. They are generally mechanically and/or chemically bound together. The open sandwich technique is usually used for Class II restorations where the underlying material forms part of the axial wall and is exposed to the oral environment (Figure 9.7).
Closed sandwich: in a closed sandwich restoration the underlying material does not come into contact with the oral cavity (Figure 9.8).
Mechanical Properties
The mechanical properties of conventional glass ionomer cements are not ideal.
• The compressive strength of the cement is adequate, just below that of dentine.
• The flexural strength is relatively low compared with other restorative materials. However, in most cases under load it is protected by the restorative.
• The compressive and flexural strengths increase with time as the cement matures.
• The solubility varies with time of exposure to the oral environment after mixing.
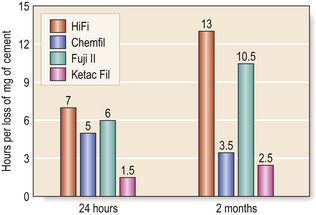
• The longer the restoration is protected from moisture, the better the resistance is to erosion (Figure 9.9).
Adhesion
• An ion exchange process where the polyacrylic acid displaces surface phosphate and calcium, enters the hydroxyapatite structure and forming a calcium polyacrylate salt. Thus an intermediate layer of calcium and aluminium phosphates and polyacrylates are formed at the tooth/restoration interface.
• A secondary bond, which is thought to occur with the collagen within the dentine, possible via hydrogen bonding.
The bond strength is relatively low (5 MPa) but appears to be durable and fit for purpose. There is considerable evidence that the bond can re-form if broken, thus it can be termed dynamic. This is due to the polyacrylate and calcium ions being in close proximity to each other.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses