Chapter 21 Alloys used in dentistry
Learning Objectives
From this chapter, the reader will:
• Be aware of the various alloys which are used in dentistry
• Understand the effects each metallic element has on the properties of these alloys
• Understand how the manufacturing processes affect and influence the dimensional stability of dental castings
• Be able to correctly prescribe an alloy for a particular indication
• Understand how alloys may be used as metal substructures to support ceramic material
• Be able to discuss the use of dental alloys in a case with a dental technician
• Know the names of currently available commercial products.
Introduction
There is a long history of the use of metals in the mouth. These materials have been demonstrated as being the most durable in the oral environment. One of the earliest metals used was pure gold. Its advantages are:
The range of applications for alloys in dentistry is far-ranging:
Alloys
Alloys may be referred to as being binary, ternary or quaternary. This means they have two, three or four metallic constituents, respectively (compare with amalgam; see Chapter 6).
Structure of alloys
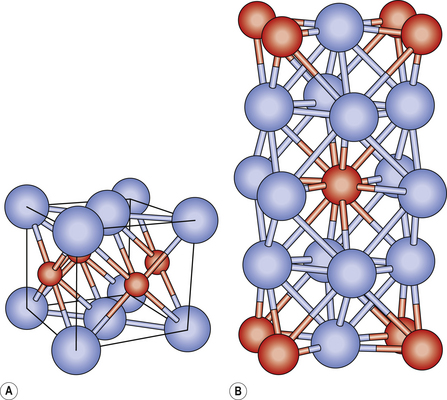
Alloys are essentially crystalline in structure. The crystals that initially form then grow towards each other until they touch. This is similar to how ice crystals form. At the point where the crystals touch, the water is fully frozen. In the same way, the metallic crystals grow as the alloy cools (Figure 21.1). The arrangement of the crystals depends on the size of the atoms of the various constituent metals. If these are similar, then atoms of one constituent can replace those of another. If one metal’s atoms are much smaller, they may be trapped between the larger atoms, filling the interstitial space between the crystals.
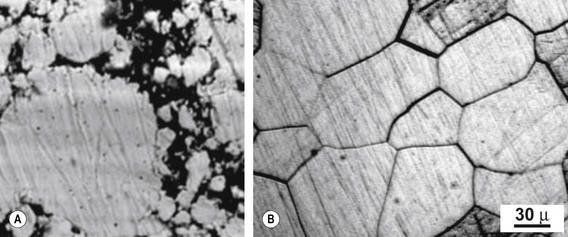
The crystalline structure consists of crystals or grains abutting one another. The boundaries between the grains are referred to as grain boundaries (Figure 21.2). The size of the grains determines the properties of the alloy. The smaller the grains the better, as more boundaries prevent dislocations in the structure. To achieve this, some elements such as iridium or ruthenium may be added to dental alloys, particularly gold-based alloys, to reduce the grain size. These elements are called grain refiners.
General mechanical properties
Strength
• Yield strength is the force per unit area (stress) required to permanently deform the alloy. Exceeding the yield strength is clearly undesirable for dental applications. Yield strength is therefore a property used to describe the behaviour of an alloy. It is measured in mega pascals.
• The yield point is defined in as the stress at which a material begins to deform plastically. Before the yield point the material will deform elastically returning to its original shape when the stress is removed. Once the yield point is passed a proportion of the deformation will be permanent and irreversible.
• Related to yield strength is hardness which increases as yield strength increased. This gives the dentist and dental technician an indication of the difficulty to grind and polish an alloy. Some metal alloys may be heat treated to increase their hardness.
• Ductility is the ability of an alloy to deform under tensile stress. This is important when clasps require to be bent and inlays burnished to enhance their fit and marginal adaptation. The stiffness of the alloy is determined by its elastic modulus and the design of the casting.
Biocompatibility
Tarnish: a thin layer of corrosion forming on the surface of metals such as copper, brass, silver, aluminium and other similar metals as a result of the surface undergoing a chemical reaction. Tarnish is not necessarily the sole result of contact with oxygen in air. Silver needs hydrogen sulphide in order to tarnish. Tarnish appears as a dull, grey or black film or coating over metal. It is a self-limiting surface phenomenon unlike rust. The outer layer of the metal reacts and the tarnish coating seals and protects the underlying layers from further reaction.
Types of alloy
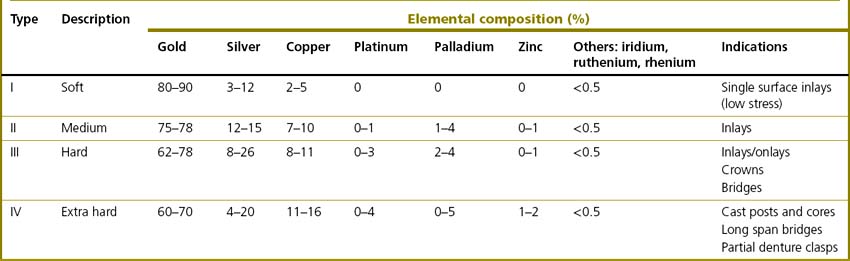
The metals used in dental alloys may be divided into two categories: noble and base metals. Examples of noble metals are gold, platinum, rhodium, ruthenium, iridium and osmium. Such elements are good for dental use as they are resistant to corrosion in the hostile environment of the mouth. From a chemistry perspective, silver is a noble metal but as far as dentistry is concerned it is not considered so because it corrodes in the mouth. These preceding elements are sometimes referred to as precious metals as they tend to be expensive. This term can be confusing as it does not refer solely to cost and therefore should be used carefully. Equally it does not mean noble as in noble elements, as silver and palladium are not dental precious metals. The term is more descriptive of the physical properties of the alloy. Nobility of the alloy depends on the sum of the amount of noble elements contained in it. The American Dental Association has defined alloys as high noble, noble and base metal alloys (Table 21.1).
Table 21.1 Definition of high noble, noble and base metal alloys according to percentage of noble metals present
| Type of alloy | Noble metal content |
|---|---|
| High noble | Contains at least 40% by weight gold and at least 60% by weight of the noble metal elements (gold, iridium, osmium, platinum, rhodium) |
| Noble | Contains more than or equivalent of 25% by weight noble metals |
| Base metal | Contains less than 25% by weight of noble meals |
Casting alloys for tooth restorations
Some cast restorations such as inlays, onlays, some crowns and bridges are composed solely of metal (Figure 21.3). The vast majority of these restorations are constructed out of noble alloys but in certain situations the clinician may prescribe the use of a base metal alloy. Both these types of alloy may also be used for bonding to dental ceramic to construct tooth-coloured restorations. To optimize the union between the alloy and ceramic, the constituents of these alloys may be varied (see later).
High noble and noble alloys
The vast majority of noble alloys are based on gold (Box 21.1). As mentioned earlier, pure gold is too soft to be used alone in dentistry and to achieve adequate mechanical properties it must be alloyed with other elements (see Table 21.2). The four types of gold casting alloy used in dentistry are summarized in Table 21.3.
Box 21.1 Measuring gold content
• Gold content of an alloy may be measured in carats.
• A carat is the percentage of gold multiplied by 24 over 100.
• Pure gold is 24 carat so a gold alloy which is 50% gold is 50%Au/100 × 24 = 12 carat.
• Gold content may also be expressed by its fineness. This is the percentage of gold multiplied by 10. Pure gold is therefore 1000 fine.
Table 21.2 Elements that are alloyed with gold for use in dentistry and the effects they impart to the final alloy
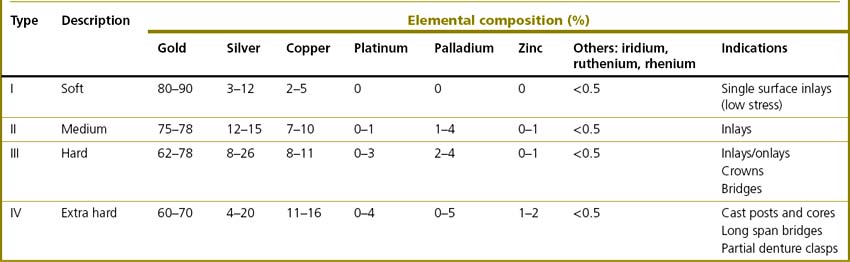
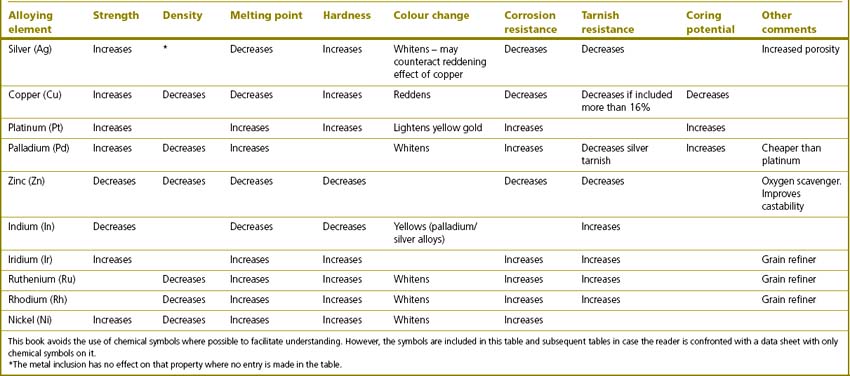
The properties of noble alloys vary markedly and this is affects their indications:
Addition of copper: order hardening
The increase in hardness is accompanied by a decrease in ductility and corrosion resistance. The element mainly responsible for this is copper. Copper conveys order hardening to the alloy. This is where the copper atoms form ordered clusters instead of being randomly distributed within the alloy. This ordered atomic structure prevents movement or slippage of the layers of atoms. For this phenomenon to occur the alloy must contain at least 11% copper and so some effect will be seen in type III gold alloys although it is seen more so with type IV. The amount of copper added works only up to a point as the alloy will tarnish if it contains more than 16% copper. Order hardening may be achieved by heating the alloy to 400 °C and holding it in the furnace at this temperature for 30 minutes.
Other constituents
Zinc is included as a scavenger of oxygen as it will preferentially react with oxygen so preventing oxidation of the other components. It is relatively reactive and pure zinc will take up oxygen to passivate the surface. It is included in noble metal alloys for the same reason as in dental amalgam (see Chapter 6).
Indications
There are several indications for prescribing a cast gold restoration:
• Gold alloys are very strong in thin section. This means that the dentist may consider providing a gold restoration where there is little interocclusal clearance. More tooth tissue may be conserved as it need not be sacrificed in favour of accommodating the dental material. The minimum thickness of a gold alloy should be 1 mm and 1.5 mm over a functional cusp.
• Cast gold restorations function well in the mouth as their wear resistance is the same as enamel; thus differential wear will not occur on opposing teeth.
• They are durable in function and have a good longevity.
• Gold alloys are dimensionally very accurate as little change occurs in this respect during their construction using the lost wax technique. This minimizes chairside time as less adjustment should be required at the fit appointment.
• If any adjustment is required at the chairside, gold alloys may be relatively easily polished by the dentist prior to fitting. Unlike ceramic, the gold restoration does not need to be returned to the dental laboratory to be finished should any chairside adjustment be required.
• The patient may elect to have a gold restoration for a variety of reasons: the use of gold to restore anterior teeth is more popular in some cultures, or on the recommendation of their dentist for one or more of the reasons listed above.
Contraindications
• The primary dental disease should be under control and stable, that is the patient’s caries rate/risk must be low and their oral hygiene good. Therefore those patients who have a high caries rate and are unable (or unwilling) to maintain a good level of oral hygiene are unsuitable for gold alloy restorations.
• Gold alloy restorations may be contraindicated in some patients on grounds of cost. To have a gold restoration prepared, constructed and fitted requires a minimum of two surgery appointments and a laboratory bill. The price of gold, even at a low level, can be considerable.
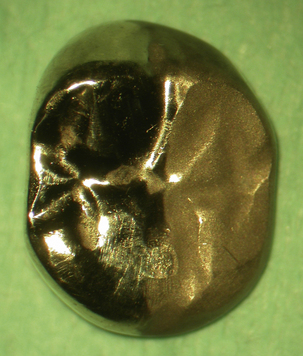
• Many patients decline gold restorations as they do not like the appearance of gold and may prefer a tooth-coloured restoration. This problem can be overcome by sandblasting the ‘polished’ surface of the gold, which has the effect of decreasing the shine or ‘glint’ of the gold. This may be a satisfactory solution for some patients (Figure 21.4).
Bonding gold alloys to tooth tissue
Restorations constructed out of gold alloys are usually luted into or onto the preparation. Gold alloy itself has no inherent ability to chemically bond to tooth tissue. However, it may be treated so that it can bond to tooth tissue with the use of an adhesive resin-based cement. If the gold alloy contains more than 16% copper, it may be heat treated by putting it in the furnace at 400 °C for 9 minutes. This forms a surface oxide layer of copper oxide, to which the resin based adhesive may bond (Figure 21.5).
Alternative metal alloys used for metal crowns
Chemical constituents of alternative metal alloys and their functions
• Common alloys used as an alternative to those containing gold are the silver-palladium and silver-platinum-copper alloys. These usually contain 60–70% silver, 25% palladium and up to 15% copper.
• A nickel-chromium or cobalt-chromium alloy may also be used as a cheaper alternative.
Properties
Base metal alloys are harder to adjust, finish and polish due to their hardness and lack of ductility. Many dental technicians sandblast the casting to remove any residual investment material and the green oxide layer. This may help to reduce the surface roughness. Electrolytic polishing may be used in preference to polishing and finishing these alloys by traditional means (see Chapter 19). However, many technicians believe that base metal alloys may be finished as well as noble alloys even though it takes longer to achieve and requires more work!
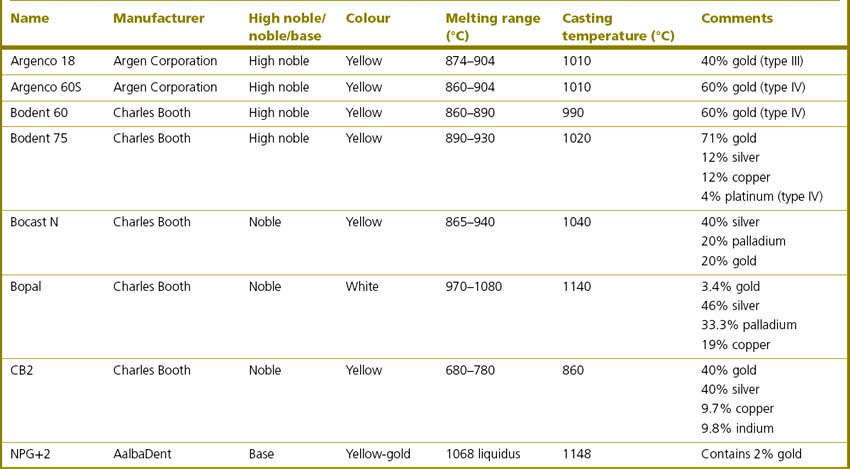
Commercially available products
Table 21.4 show some commonly used casting alloys currently available on the market. It is clear from Table 21.4 that alloys of different composition can have similar melting ranges and casting temperatures. Care needs to be exercised in their selection. It is wise to establish a dialogue between dentist and technician so that the dental team can determine which alloy should be used in any particular case.
Table 21.4 Some commonly used casting alloys of high noble, noble and base metal alloys currently available on the market
Alloys are usually supplied to the dental technician as ingots (Figure 21.7).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses