Chapter 17 Preventive and periodontal materials, implants and biomaterials
Learning Objectives
From this chapter, the reader will:
• Be aware of the many preventive products available on the market and be able to prescribe them appropriately
• Have a knowledge of the products which aid in the diagnosis of dental diseases or help identify at-risk patients
• Be aware of the materials used in periodontics, such as local antimicrobials and regenerative materials
• Understand the rationale behind the use of these materials and how to use them to best effect
• Have a working knowledge of the various oral surgical materials
• Understand the materials used in relation to dental implants
• Know the names of currently available commercial products.
Introduction
The old adage of prevention being better than cure is never truer than in dentistry. As dental tissues do not repair themselves readily, preventive dentistry has a critical role. Many of the vast range of products available on the market have a dual role, in that they are to prevent both dental caries and periodontal disease. This is mainly achieved by the control of dental plaque, by breaking down the biofilm and the killing of microorganisms. However, they may also perform additional functions, for example having a desensitizing effect on hypersensitive teeth. It is therefore not possible to categorize neatly these multifaceted products in a convenient way.
Preventive Materials
Toothpastes
• Removing food debris, plaque, pellicle and any extrinsic stain
• Polishing the surface of the tooth to reduce the risk of adherence of debris
• Providing a means of delivering various forms of therapeutic agent.
Composition
The main ingredient is some form of abrasive, which will mechanically remove debris and also help in the polishing process. Toothpastes may contain some form of detergent to aid the cleaning process and improve the wetting of the surface of the teeth. Therapeutic agents such as one of the various forms of fluoride and whitening agents are added to enhance the enamel surface. Calculus (tartar) control agents such as sodium pyrophosphate and potassium pyrophosphate are also incorporated as well as desensitizing agents such as strontium chloride, potassium nitrate and calcium sodium phosphosilicate. See Table 17.1 for a list of common toothpaste ingredients.
More recently there have been attempts to add antimicrobial agents such as triclosan, an antibacterial and antifungal agent that is effective in preventing gingivitis (Figure 17.1). Chemically, triclosan is a polychlorophenoxyphenol. These antimicrobial agents help to control bacterial growth by preventing the bacterial proliferation phase of plaque development. Many dentists recommend a toothpaste to their patients which contains fluoride, triclosan and some form of pyrophosphate for the reasons mentioned above and described in detail later.
Flavouring agents may also include saccharin and xylitol. These are sugar substitutes, and xylitol is associated with caries inhibition. A variety of tooth-whitening agents may also be added. These are generally based on peroxide chemistry. For further details of these bleaching agents, see Chapter 18.
The factors which influence the effect of a toothpaste on the teeth are shown in Box 17.1.
Box 17.1 Factors influencing the effect of a toothpaste on the teeth
Fluoride agents
Stannous fluoride is frequently used in combination with sodium hexametaphosphate, providing 1100 ppm fluoride (Figure 17.2). Stannous fluoride has been demonstrated to
• Strengthen the enamel by inhibiting demineralization and promoting remineralization
• Stop the multiplication of Streptococcus mutans (one of the bacteria implicated in the development of dental caries).

Fig. 17.2 A toothpaste containing stannous fluoride and sodium hexametaphosphate (Pro-Expert, Oral B).
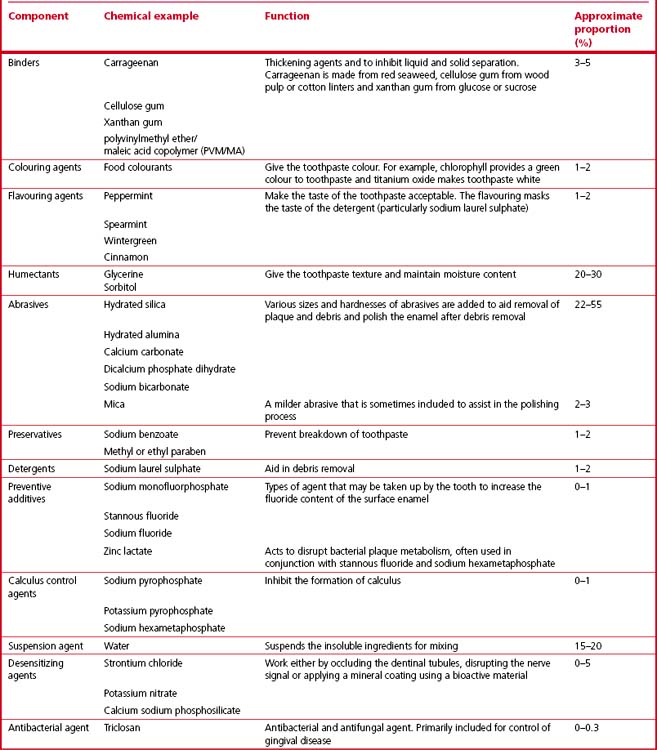
Sodium monofluorophosphate has been used as an alternative as it is more stable and not so susceptible to degradation. Most currently available toothpastes use either 0.45% stabilized stannous fluoride or the equivalent sodium fluoride concentration of 0.24%. Both deliver 1100 ppm fluoride. As well as the well-proven success in reducing the incidence of caries, both these materials also appear to have some beneficial effect on gingival health. Higher levels of fluoride are provided in certain toothpastes, which are recommended for patients with a high caries incidence. These can produce the equivalent of 2400 and 5000 ppm fluoride (Figure 17.3).
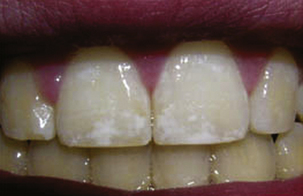
They should be used with care as overzealous use can potentially lead to enamel fluorosis (Figure 17.4) if too much is swallowed. Similarly some toothpastes are available which contain a lower concentration of fluoride and are intended for use by children and infants (Figure 17.5).
Commercially available products
Table 17.2 and Figure 17.6 show representative examples of commercially available toothpastes. It is apparent that many international pharmaceutical companies sell a variety of toothpastes under different brand names. Many are claimed to carry out similar processes.
Table 17.2 Some toothpastes currently available on the market
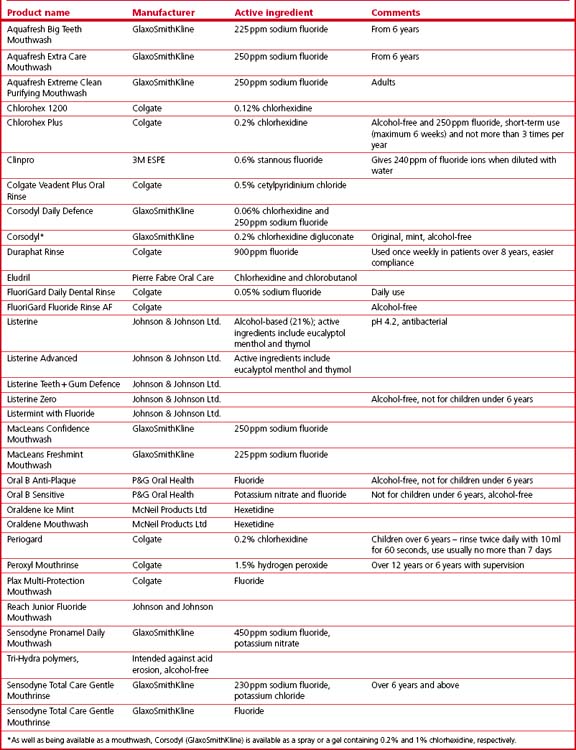
| Product | Manufacturer | Comments |
|---|---|---|
| Aquafresh Big Teeth | GlaxoSmithKline | 6 + years; 1400 ppm fluoride |
| Aquafresh Fresh ‘N’ Minty | GlaxoSmithKline | |
| Aquafresh ISO Active | GlaxoSmithKline | |
| Aquafresh Little Teeth | GlaxoSmithKline | 4–6 years and above, 1000 ppm fluoride |
| Aquafresh Milk Teeth | GlaxoSmithKline | 0–3 years and above, 500 ppm fluoride |
| Arm and Hammer Advanced Whitening | Arm and Hammer | Baking soda base with micropolishing abrasives to polish the surface of the teeth |
| Arm and Hammer Enamelcare Whitening | Arm and Hammer | Contains calcium phosphate-based remineralizing system |
| Arm and Hammer Original Coolmint | Arm and Hammer | Abrasive is baking soda, so is less abrasive |
| Arm and Hammer Enamel Care Sensitive | Arm and Hammer | Addition of calcium phosphate-based agent to coat the foot surface |
| Colgate Proclincal White | Colgate | 0.24% sodium fluoride |
| Colgate Cavity Protection | Colgate | 0.76% sodium monofluorophosphate |
| Colgate Total Advanced clean | Colgate | Abrasive is silica |
| Colgate Oxygen Pure Freshness | Colgate | |
| Colgate Sensitive Pro-Relief | Colgate | 1450 ppm as sodium monofluorophosphate |
| Colgate Smiles Toothpaste | Colgate | |
| Colgate Total Gum defence | Colgate | Triclosan copolymer added |
| Corsodyl Daily | GlaxoSmithKline | 0.31% sodium fluoride (1400 ppm) |
| Crest | P&G Oral Health | |
| Crest Complete | P&G Oral Health | |
| Duraphat 2800 ppm | Colgate | Over 10 years (POM) |
| Duraphat 5000 ppm | Colgate | Over 16 years (POM) |
| Macleans White and Clean ISO-active | P&G Oral Health | |
| Mentadent P | Unilever | |
| Oral-B Children’s Toothpaste | P&G Oral Health | 2–4 years of age |
| 5–7 years of age | ||
| Oral B Pro Expert | P&G Oral Health | Over 12 years, stannous fluoride |
| Pronamel Sensodyne | GlaxoSmithKline | |
| Retardex | Rowpar Pharmaceuticals Inc. | |
| Rembrandt Plus | P&G Oral Health | |
| Rembrandt Sensitive | P&G Oral Health | |
| Sensodyne Original | GlaxoSmithKline | Fluoride free |
| Sensodyne Rapid Relief | GlaxoSmithKline | |
| Sensodyne Toothpaste Gel | GlaxoSmithKline | POM |
| Sensodyne Total Care Extra Fresh | GlaxoSmithKline | |
| Sensodyne Whitening | GlaxoSmithKline |
POM, prescription only medicine. These toothpastes therefore should be prescribed by a dentist or doctor.
Mouthwashes
Chlorhexidine
Chlorhexidine gluconate is used extensively to treat periodontal diseases. It has already been discussed in Chapter 13 as many dentists use it during the chemo-mechanical preparation of the root canal system. It is widely regarded as the gold standard active agent in periodontal treatment. It is used in a 0.2% solution and it is bacteriostatic as it inhibits growth of plaque bacteria. It has excellent substantivity as it remains active for up to 12 hours after the first application.
Unfortunately, chlorhexidine has some disadvantages:
• Its main drawback is that is stains teeth quickly because it is adsorbed onto the tooth surface and chemicals which stain are attracted to it. This is more pronounced with certain substances such as tea, coffee and red wine. The margins of resin-based composites may stain as will any rough surfaces. If this occurs then care is required when removing the stain that no damage to the resin composite restoration ensues. If the product is used on a toothbrush rather than as a mouthwash then the staining and unpleasant taste is reduced. To facilitate this, a gel presentation is available (Figure 17.7). Should staining occur, it is reasonably easily removed by a professional cleaning (see Chapter 19 for information on products used for this purpose).
• Its efficacy may be compromised by certain other chemicals added to products. Chlorhexidine is cationic in nature. Anionic compounds, particularly surfactants, such as sodium lauryl sulphate which is used in toothpaste to reduce staining, reduces the efficacy of chlorhexidine.
• It has a very poor penetration of the subgingival biofilm and so its use in anaerobic environments like deep periodontal pockets is limited. Furthermore, it is flushed out from the pockets by crevicular fluid too quickly and neutralized by inflammatory products such as immunoglobulins.
• It has a taste which is very difficult to mask by other chemicals, so preparations containing chlorhexidine may not have a pleasant taste. Many patients complain that it has a slightly metallic taste. Attempts to improve the taste at 0.2% have affected the efficacy.

Fig. 17.7 A gel containing chlorhexidine digluconate, which may be applied to the teeth with a toothbrush.
Other fluoride products
Fluoride gels
Another gel product, Cervitec Gel (Ivoclar Vivadent) combines fluoride in the form of 900 ppm sodium fluoride and 0.12% chlorhexidine (Figure 17.9). It is indicated to protect exposed root surfaces, treat hypersensitive areas and reduce the bacterial load on the surface of the tooth. It has been shown in clinical trials to be useful to reduce the caries rate in children.
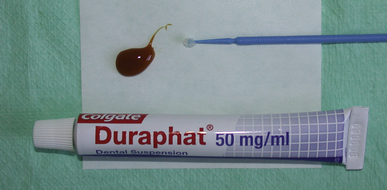
Fluoride varnishes
The best-known fluoride varnish is Duraphat (Colgate; Figure 17.10). This banana-flavoured varnish is presented in a tube or in ampoules for ease of application.
![]()
Some fluoride varnishes contain colophony. This is another term for rosin (see Chapters 12 and 16). Patients may have hypersensitivity to colophony and so the dentist should enquire about this when taking the medical history if one of these products is being considered for use. Colophony is also contraindicated in patients with bronchial asthma.
Desensitizing agents
• Desensitizing the nerve ending
• Mechanically occluding the dentinal tubules to preventing fluid movement.
There are many different presentations of desensitizing products:
The mode of action of desensitizing toothpastes and mouthwashes has been discussed earlier.
Desensitizing varnishes
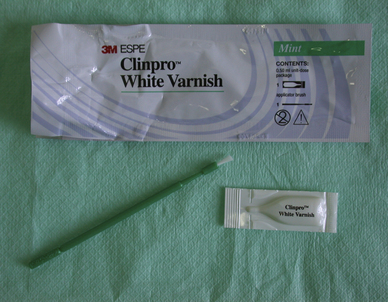
Fluoride varnishes are frequently used by dentists to treat dentinal hypersensitivity. The main indication is to apply high levels of fluoride to a particular surface in an attempt to arrest an early carious lesion. Varnishes are also very effective at reducing dentinal hypersensitivity. Probably the most commonly used product for this indication is Duraphat (Colgate), however, other varnishes are now available which are intended specifically for the treatment of dentinal hypersensitivity. One such product is Clinpro White Varnish (3M ESPE; Figure 17.11), which also contains fluoride. In most cases, the desensitizing effect is achieved by the occlusion of the tubules and remineralization of the surrounding tooth tissue.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses