Chapter 8 The tooth-coloured restorative materials II
Compomers
Learning Objectives
From this chapter, the reader will:
• Understand what compomers are
• Appreciate the significance of their constituents and how these influence the properties of the material
• Understand how the constituents affect the material’s clinical performance
• Have an increased appreciation of how to use this type of material more effectively
• Be aware of the wide spectrum of usages and know the names of currently available commercial products.
Introduction
Compomers first appeared in the early 1990s in an attempt to combine the potential advantages of fluoride release as seen with the glass ionomer cements, with the many advantages of the resin composites. The aim was therefore to produce a resin composite which exhibited a sustained and effective release of fluoride. Previous attempts to achieve this had involved adding various fluoride salts to conventional resin composite formulations, but this proved to be less than successful. The mechanism of fluoride release relies on water being absorbed by the resin composite which then dissolves fluoride salts such as ytterbium fluoride followed by diffusion of fluoride ions out of the material. However, the nature of the resin composites meant that only the fluoride in the subsurface layers was released by this process because of the difficulty encountered by the fluoride ions in diffusing through the bulk of polymerized resin. Small amounts of fluoride were released initially but this was not sustained in the longer term. Unfortunately, another effect of these additions was to reduce the resin composite’s longevity because of degradation of the methacrylates.
Nomenclature
The commonly accepted name for these materials is ‘compomer’, which was coined by Dentsply, the manufacturer of Dyract, the first material of this type (Figure 8.1). However the generic name for this material is polyacid-modified resin composite, which effectively describes their composition and, by implication, their setting mechanism.
Composition
The material is essentially a composite in nature, in that it is composed of a resin components and a filler (Table 8.1).
| Component | Chemical example | Reason for inclusion |
|---|---|---|
| Filler | Fluoro-alumino-silicate glass | Imparts strength |
| Source of fluoride ions for use in the secondary reaction | ||
| Dimethacrylate monomer | Urethane dimethacrylate (UDMA) | Primary monomer forming the resin matrix |
| Difunctional resin | TCB resin: the reaction product of butane tetracarboxylic acid and hydroxyethyl methacrylate | Cross-linking agent in the primary reaction |
| Source of carboxyl groups for secondary reaction with glass | ||
| Photo-activator and initiator | Camphorquinone | Required to effect light polymerization |
| Tertiary amine | ||
| Hydrophilic monomers | Glycerol dimethacrylate | Enhances water diffusion within resin matrix |
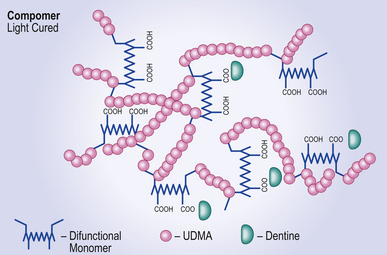
Resin component
The resin part has two monomeric components:
• A dimethacrylate, usually urethane dimethacrylate (UDMA) and
• A difunctional resin monomer, which has both carboxyl and methacrylate groups. Another type of resin which may perform in the same way is the methacrylated polycarboxylic acid found in some resin-modified glass ionomer cements (see Chapter 10).
Setting Reaction
The setting reaction is a two-stage process.
Stage 1
When the clinician is satisfied with the material’s placement in the cavity, the initial setting reaction is initiated by light as with the composite resins. This is a free radical polymerization reaction that leads to cross-linking of the end groups on the UDMA and the methacrylate groups on the difunctional resin to form a resin polymer matrix in which the glass is trapped (Figure 8.2).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses