8
MTA Root Canal Obturation
George Bogen,1 Ingrid Lawaty,2 and Nicholas Chandler3
1 Private Practice, USA
2 Private Practice, USA
3 Faculty of Dentistry, University of Otago, New Zealand
INTRODUCTION
Mineral trioxide aggregate (MTA) was originally introduced to dentistry as a root-end filling and perforation repair material (Lee et al. 1993; Abedi & Ingle 1995; Torabinejad & Chivian 1999). This hydraulic calcium-silicate based cement has been shown to exhibit excellent biocompatibility and pronounced osteoconductivity. The therapeutic scope of this bioactive cement has expanded to include pulp capping, pulpotomy, apexogenesis, repair of anatomical anomalies, resorptions, regenerative procedures, and recently as a canal obturation material (Torabinejad & Chivian 1999; Koh et al. 2001; O’Sullivan & Hartwell 2001; White & Bryant 2002; Branchs & Trope 2004; Aggarwal & Singla 2010; Roig et al. 2011; Dreger et al. 2012). Since perforation repair, root-end filling and apical plug placement represent a form of partial canal obturation, the complete filling of canal systems with MTA can also be viewed as an advanced clinical concept that provides significant advantages in surgical, conventional or combined therapies.
MTA canal obturation offers an innovative method to approach challenging endodontically involved teeth that may not respond using traditional filling materials and sealers when extensive pathosis is present. Although gutta-percha (GP) is the most universally employed core material in conventional root canal therapy, it is susceptible to bacterial ingress when exposed directly or indirectly to oral fluids (Swanson & Madison 1987; Madison & Wilcox 1988; Khayat et al. 1993; Jacobson et al. 2002; Yazdi et al. 2009). Furthermore, the ability of all obturation materials to maintain a bacteria-tight seal and prevent recontamination is largely dependent on the sealing ability and quality of the provisional and permanent coronal restorations (Saunders & Saunders 1994; Ray & Trope 1995; Uranga et al. 1999; Tronstad et al. 2000; Siqueira et al. 2000; Balto 2002; Weston et al. 2008). Many dentists would claim a key advantage of GP is its ease of removal during retreatment. This unusual quality of the material has allowed improved success rates for root canal retreatment along with the evolution of instrumentation and visualization. However, it may be advantageous to use a canal filling material that can overcome the many shortcomings of GP based systems.
In general, the ideal root filling material should preclude reinfection of the root canal system by eliminating the microbial nutrient supply and creating an inhospitable environment for their continued survival (Sundqvist & Figdor 1998; Carrotte 2004). Its ideal requirements therefore include that it be nonirritating to periapical tissues, bacteriostatic, radiopaque, sterile, nonstaining, insoluble, not affected by tissue fluids, dimensionally stable, seal laterally and apically, exhibit biocompatibility, and be easily placed and removed (Grossman 1982). If a material has the ability to completely seal the canal system apically and laterally, then its removal should be difficult or virtually impossible (Torabinejad et al. 1993; Boutsioukis et al. 2008). Although MTA and all other obturation materials may not be ideal in this regard, MTA can provide important advantages due to its bioactive and bioinductive properties.
Some clinicians view MTA obturation as a relatively new approach to orthograde canal treatment (O’Sullivan & Hartwell 2001; de Leimburg et al. 2004; D’Arcangelo & D’Amario 2007; Bogen & Kutler 2009). However, this filling method is an older concept and is antedated by a German publication from the late nineteenth century. During that period, Portland cement, a material very similar to MTA, was not only used to fill root canals but also placed as a pulp capping agent (Witte 1878; Schlenker 1880). In Mayan populations, around 400 ce a related substance was used as an inlay cement (Versiani et al. 2011). Interestingly, the early reports of root fillings show that the material was quite successful in curing “apical periostitis”, with patients exhibiting diminishing or resolution of pain after treatment and during healing. Both the early Portland cement investigators claimed that they knew of no failures during the observation period, and commented that in replantation cases it also resulted in successful canal closure. They remarked that the material when set produced a “porous” seal, which bound well with other cement filling materials and that the only notable drawback was discoloration of anterior teeth.
Since the nineteenth century, the materials used to obturate the cleaned and shaped root canal space have undergone considerable modification as our understanding of the etiology and microbiology of endodontic disease has expanded. Materials included pastes, cements, plastics and solids (Grossman 1982). Gutta-percha transitioned into popularity as the material of choice, based on its ease of delivery, availability and biocompatibility (Weine 1992; Glick & Frank 1986; Seltzer et al. 2004). A wide range of core materials have since been introduced that share common properties with GP.
Research investigations have shown a variety of recently developed obturation materials to have acceptable sealability and improved handling properties when used in conjunction with sealers to fill the root canal system. The different compositions of these core materials can include epoxy resin, glass-ionomer, bioceramic, methacrylate, synthetic polyester, and silicone-based substances. Some investigators have recommended filling canals with resin or silicone-based sealers alone rather than a core and sealer combination (Malagnino et al. 2001; Tanomaru-Filho et al. 2007; Guess 2008; Cotton et al. 2008; Ordinola-Zapata et al. 2009; Williamson et al. 2009; Hammad et al. 2009; Ari et al. 2010; Kato & Nakagawa 2010; Savariz et al. 2010; Pameijer & Zmener 2010; Pawińska et al. 2011; Anantula & Ganta 2011; McKissock et al. 2011). The current search for new filling materials indicates that even though GP is currently the most widely accepted obturation substance, major advances are needed to satisfy the requirements of the ideal material including MTA.
MTA obturation can offer distinct advantages by promoting the repair of the periodontium and the supporting tissues of the tooth (Pitt Ford et al. 1995; Zhu et al. 2000; Holland et al. 2001; Zhang et al. 2009). The induction of cellular repair responses by a filling material that can promote cementum deposition, encourage bone formation and periodontal ligament regeneration must be considered a substantial breakthrough when treating teeth compromised by long-standing disease and failed conventional treatments. The specific characteristics that contribute to the bioactive properties of MTA will be reviewed in order to understand the advantages that this hydraulic tricalcium silicate cement can provide.
CHARACTERTICS/PROPERTIES
Mechanisms of action in obturation
Several key features in the composition of MTA account for its bioactive and bioinductive properties when used as a canal filling cement. These include: the material’s particle size, hydration products, sustained pH, interstitial layer formation with dentin, sealing ability, setting expansion, and antimicrobial properties (see Table 8.1).
Table 8.1 Advantages of MTA as a root filling material.
| Sustained alkaline pH during slow set Forms interfacial layer with dentin similar to hydroxyapatite Seals at zero microns when examined under SEM Particle size penetrates/occludes dentinal tubules Setting properties not affected by smear layer Inhibits growth of E. faecalis and C. albicans Promotes cementogenesis and PDL reformation Promotes bone coupling factors and osteogenesis Antibacterial effect is enhanced in presence of dentin Can increase resistance of root to fracture |
Particle size
Several investigations have analyzed the particle size and shape of ProRoot MTA, in its gray (GMTA) and white (WMTA) formulations (Lee et al. 2004; Dammasckhe et al. 2005; Camilleri et al. 2005; Camilleri 2007; Asgary et al. 2006; Komabayashi & Spångberg 2008a). Overall, it may be said that the particle size of WMTA is finer and more homogeneous than GMTA. Komabayashi and Spångberg reported that the diameter of GMTA particles measured 10.48 ± 5.68 µm in the low-power-field (LPF) mode, and 3.05 ± 2.44 µm at high-power-field (HPF) mode. The diameter of WMTA particles has been measured at 9.86 ± 4.73 µm at LPF mode and 2.96 ± 2.36 µm at the HPF. WMTA therefore contains smaller particles with a narrower range of size distribution. Approximately 70% of both GMTA and WMTA exhibit an average particle size of 1.5 to 3.0 µm. The investigation demonstrated that the small particles permit their entry into open dentin tubules, which average 2–5 µm in diameter (Garberoglio & Brännstrom 1976). This may constitute a mechanism for hydraulic sealing (Komabayashi & Spångberg 2008b). The size and shape of MTA particles may therefore facilitate the occlusion of tubules where microorganisms may persist and otherwise survive following the cleaning and shaping of the root canal system.
Hydration products and pH
MTA releases calcium ions during its setting reaction and more importantly provides an alkaline pH (Holland et al. 2002; Lee et al. 2004; Santos et al. 2005; Bozeman et al. 2006; Camilleri 2008a). The sustained alkaline pH of 12.5 is a potent antibacterial and antifungal property (Duarte et al. 2003; Al-Nazhan & Al-Judai 2003; Fridland & Rosado 2005; Al-Hezaimi et al. 2006a). It has also been demonstrated that MTA leaches calcium ions several days after the initiation of hydration and during setting (Ozdemir et al. 2008). A further reaction during hydration produces a high-sulfate calcium sulfoaluminate (Taylor 1997; Budig & Eleazer 2008). The continuous release of calcium from the setting MTA diffuses through dentinal tubules, with increasing calcium ion concentration during the prolonged curing process (Fridland & Rosado 2005; Camilleri 2008a; Ozdemir et al. 2008).
It has also been postulated that the biocompatibility of the cement may be attributable to the release of hydroxyl ions and the formation of calcium hydroxide during the course of hydration (Camilleri 2008b). In a further reaction, calcium silicates react with calcium hydroxide to form calcium silicate hydrate gel, producing an alkaline environment unfavorable towards commonly encountered microorganisms such as Enterococcus faecalis (Sen et al. 1995; Molander et al. 1998; Peciuliene et al. 2001; Santos et al. 2005) and Candida albicans (Al-Nazhan & Al-Judai 2003; Mohammadi et al. 2006; Al-Hezaimi et al. 2006a). MTA has shown to be effective in bacterial elimination and to decrease bacterial viability (Ribeiro et al. 2006; Jacobovitz et al. 2009).
Formation of interstitial layer
As the setting reaction proceeds, hydroxyapatite is produced, which occurs when the calcium ions released by the MTA come into contact with tissue fluid and culminates in interstitial layer formation (Lee et. 2004; Bozeman et al. 2006). During this process, an amorphous calcium phosphate initially forms, which transforms later to an apatite phase. Calcium-deficient B-type carbonated apatite crystallites characterize this latter phase. During the process of skeletal calcification, this weak crystalline amorphous calcium phosphate is recognized as an important intermediate that precedes apatite formation (Tay et al. 2007). The spontaneous apatite formation induced through the setting activity of MTA accumulates within collagen fibrils. As the development of the interfacial layer proceeds, apatite deposition promotes mineral nucleation on the dentin surface, which is characterized by tag-like structures extending into the dentinal tubules (Reyes-Carmona et al. 2009; Okiji & Yoshiba 2009). Materials characterized by an apatite layer have been shown to form a chemical bond with calcified tissues such as bone (Holland et al. 1999a; Reyes-Carmona et al. 2009).
The adherent interstitial dentin-MTA interfacial layer that forms in the presence of tissue fluid phosphates shows marginal adaptation superior to amalgam, Intermediate Restorative Material, and Super-EBA under scanning electron microscopy (Torabinejad et al. 1993; Sarkar et al. 2005). This layer formation may be a principal factor in minimizing leakage by filling the gap along the interface and by interacting with dentin. It can also be considered a hybridized layer, since it is an intercrossed chemically bonded layer of collagen and MTA (Torabinejad et al. 1993). The layer formed resembles hydroxyapatite in structure and composition when examined by X-ray diffraction analysis (Sarkar et al. 2005; Bozeman et al. 2006). The combination of the resulting mineralized interstitial layer and the sustained, elevated pH for protracted periods (12.5 after curing) may therefore provide potent bacteriostatic and bactericidal properties, allowing improved entombment and killing of surviving microorganisms (Torabinejad et al. 1995a; Camilleri et al. 2005).
Fracture resistance
Teeth root filled with MTA have been reported to exhibit greater resistance to fracture than untreated controls (Bortoluzzi et al. 2007; Hatibović-Kofman et al. 2008). MTA may provide enhanced resistance to fracture and also contribute to strengthening of tooth structure. This characteristic of MTA has been shown in vivo after storage of extracted teeth for a period of one year (Topcuoğlu et al. 2012). The fracture resistance in MTA-treated teeth may be attributable in part to the presence of metalloproteinase-2, which has the capacity to inhibit collagen destruction (Tjaderhane 2009; Parirokh & Torabinejad 2010).
Chemomechanical instrumentation of the root canal significantly weakens dentin, making teeth more vulnerable to fracture when using conventional filling materials (Sim et al. 2001; Grigoratos et al. 2001; Topcuoğlu et al. 2012). In particular, calcium hydroxide used to disinfect root canals over extended periods can weaken immature teeth and considerably reduce fracture resistance (Andreasen et al. 2002). MTA has been shown to increase fracture resistance over time, findings that have been confirmed for MTA and other proprietary calcium silicate-based cements (Tuna et al. 2011). This feature of MTA obturation can positively influence the retention and long-term outcome for the involved tooth.
Sealing ability and setting expansion
In order to properly seal a canal and prevent bacterial penetration, the material should be dimensionally stable, adhering and adapting precisely to the dentin wall (Storm et al. 2008). The sealing capacity of MTA has been shown to be superior to that of other filling materials currently used (Torabinejad et al. 1993, 1995b; Wu et al. 1998; Roberts et al. 2008). It also resists leakage at a higher rate when placed in a moist environment (Torabinejad et al. 1995c; Gondim et al. 2003; Chogle et al. 2007). It has been postulated that this favorable characteristic may be at least in part due to the inherent property of both GMTA, and to a lesser degree WMTA, to expand during setting (Storm et al. 2008; Okiji & Yoshiba 2009; Hawley et al. 2010). The interaction of calcium ions and phosphate ions in the medium that facilitate the formation of apatite crystals at the MTA-dentin interface may contribute to this linear expansion (Sarkar et al. 2005; Tay et al. 2007; Okiji & Yoshiba 2009).
A study comparing white and GMTA as apical barriers showed GMTA to produce the superior seal (Matt et al. 2004). When challenged using human saliva it appears that GMTA is superior and that both are preferable to vertically condensed GP and sealer (Al-Hezaimi et al. 2005). Storm et al. (2008) showed that the linear expansion in water at 24 hours was 1.02% for GMTA and 0.08% for WMTA. When hydrated with Hank’s balanced salt solution, the expansion was 0.68% for GMTA and 0.11% for WMTA. There is some speculation that the linear expansion of MTA when used as an obturation material may contribute to the propagation of undetected root infractions (De Bruyne & De Moor 2008). Although the seal achieved with GMTA may be superior, when infractions are suspected or detected, WMTA may be the preferable material.
APPLICATIONS/USES
Conventional obturation
Using MTA as the filling material in uncomplicated root canals is an acceptable treatment option for conventional obturation, patients with holistic treatment concerns and cases with extensive pathosis. Teeth with external inflammatory or internal root resorption, open apices or patients seeking “alternative” filling materials may be prime candidates for MTA obturation during initial treatment. Although some clinicians may argue that MTA limits or precludes retreatment options, surgical intervention can be initiated if healing is unfavorable. The advantages of MTA when selected in initial treatment can provide remarkable outcomes without the need for surgical root-resection and related post-treatment morbidity (Kvist & Reit 2000) (see Fig. 8.1).
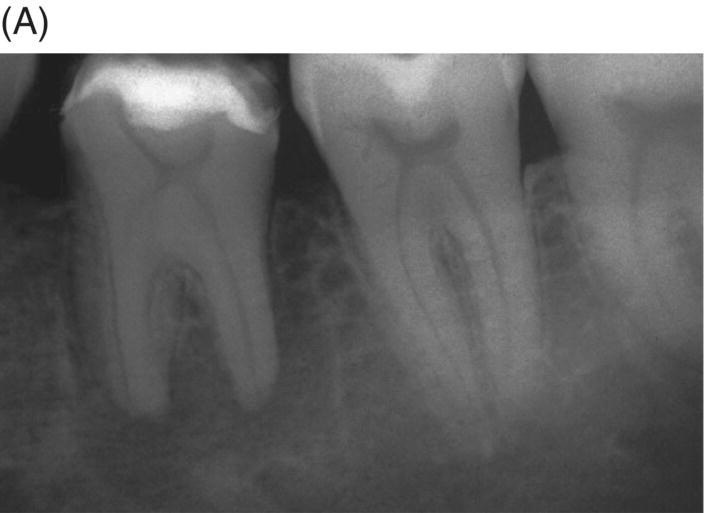
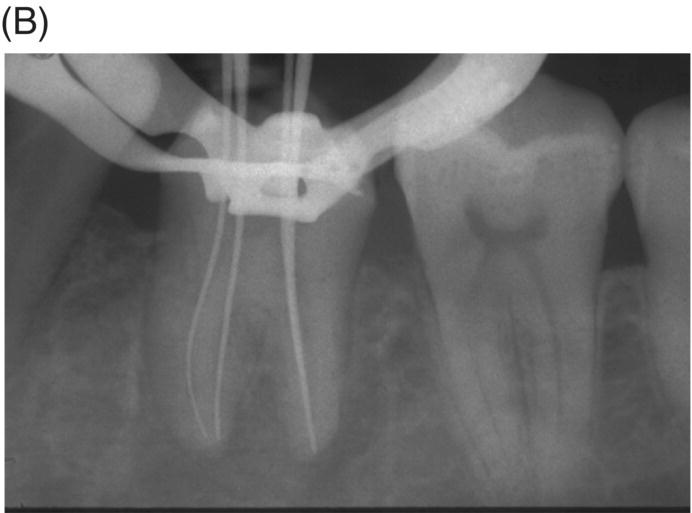
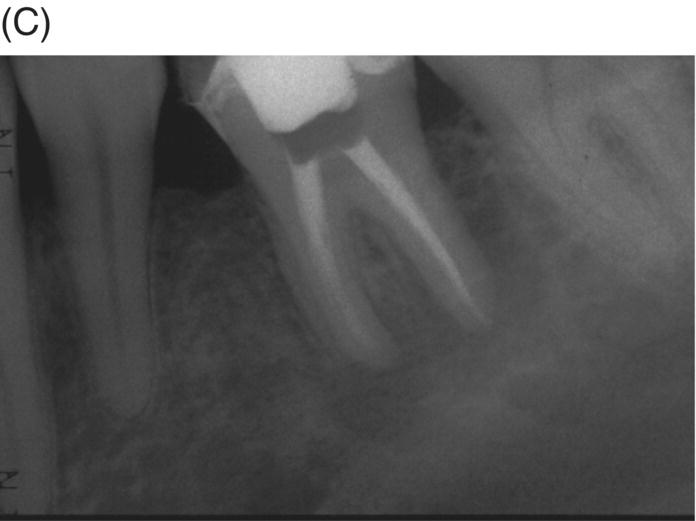
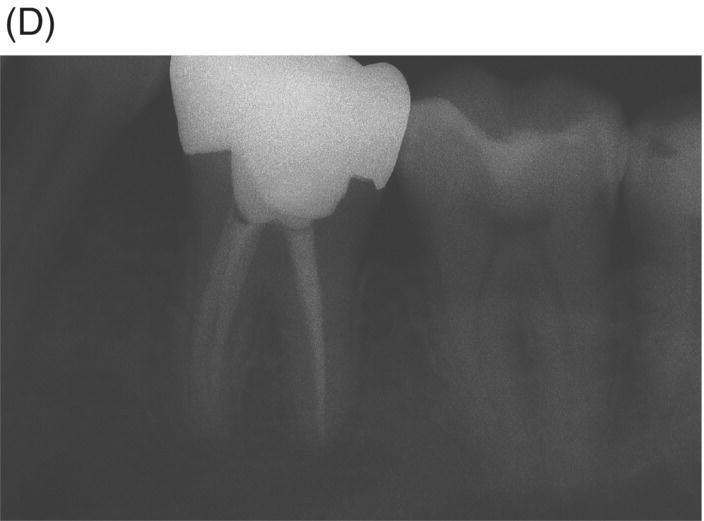
Fig. 8.1 (A) Forty-one year-old male patient with mandibular left first molar exhibiting extensive periradicular pathosis. The patient was concerned about gutta-percha and elected MTA obturation “alternative” treatment. (B) Radiographic working length confirmation. (C) MTA obturation of all three canals to the level of the pulpal floor. (D) Nine-year 8 month radiographic recall. Tooth restored with zinc phosphate cement core and full gold crown. Note absence of periradicular pathosis.
Inflammatory apical root resorption is a common complication of long-standing periapical disease (Kaffe et al. 1984; Laux et al. 2000; Vier & Figueiredo 2004). Root apices of teeth with necrotic pulps often exhibit moderate to severe foraminal and periforaminal resorption, depending on the duration of disease. Studies have identified internal apical resorption in teeth with periapical lesions in 74.7 to 81% of cases (Laux et al. 2000; Vier & Figueiredo 2002). This feature can impede healing when GP-based core materials are used, as they may not provide an adequate seal and do not promote repair. Moreover, these regions may also be vulnerable to biofilm formation from Gram-positive facultative anaerobes that can prevent periapical healing when GP is the filling material (Takemura et al. 2004; Noguchi et al. 2005). Inter-radicular biofilms are present in the majority of cases with large periapical lesions (Siqueira & Lopes 2001; Nair et al. 2005; Lin et al. 2008; Ricucci & Siqueira 2010), and they may be present in initial and refractory endodontic disease. Obturation using MTA may have an advantage in these challenging cases (Yildirim & Gencoglu 2010). Research in dog models has shown that teeth obturated with MTA feature cementum in the main canal, and in some instances the accessory canals (Holland et al. 1999b) (see Fig. 8.2).
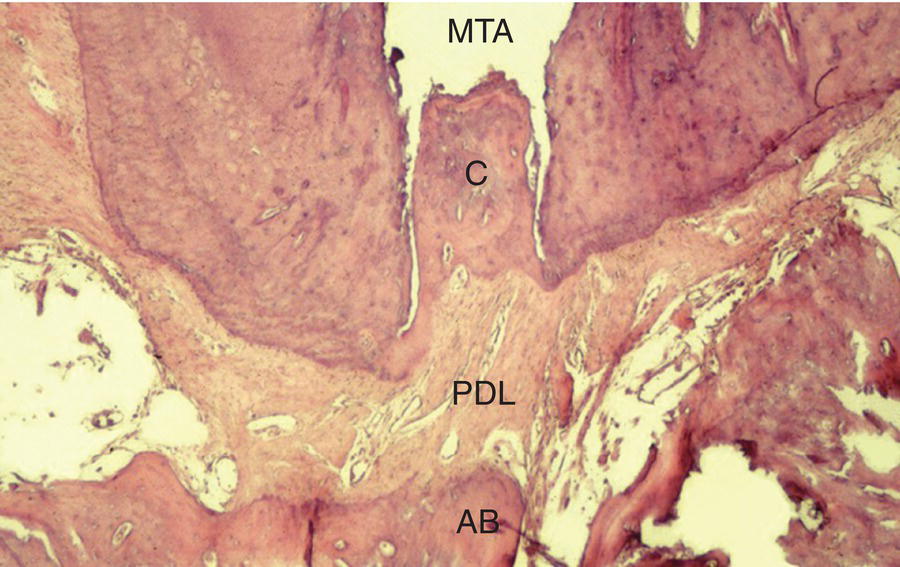
Fig. 8.2 Histological section of a dog tooth obturated with MTA at 180 days. Note biological closure of the main canal by new cementum (C), MTA plug, intact periodontal ligament (PDL), alveolar bone (AB) and absence of inflammatory cells. Hematoxylin and eosin, original magnification 100×. Courtesy of Dr. Roberto Holland, São Paulo, Brazil.
If teeth present with radiographic evidence of apical root resorption, MTA should be considered as the ideal filling material. Furthermore, when uncontrolled hemorrhage is present after cleaning and shaping, a 3–5 mm apical plug of MTA is the preferred option (Matt et al. 2004; Al-Khatani et al. 2005; Pace et al. 2008). MTA can seal internal resorptive defects and counteract the possible effects of pathogenic biofilms and bacteria harbored in dentinal tubules (Matt et al. 2004; Ricucci & Siqueira 2010). The procedure is best executed using hand instrumentation with either pluggers or hand files, as extrusion of material is possible (see Fig. 8.3).
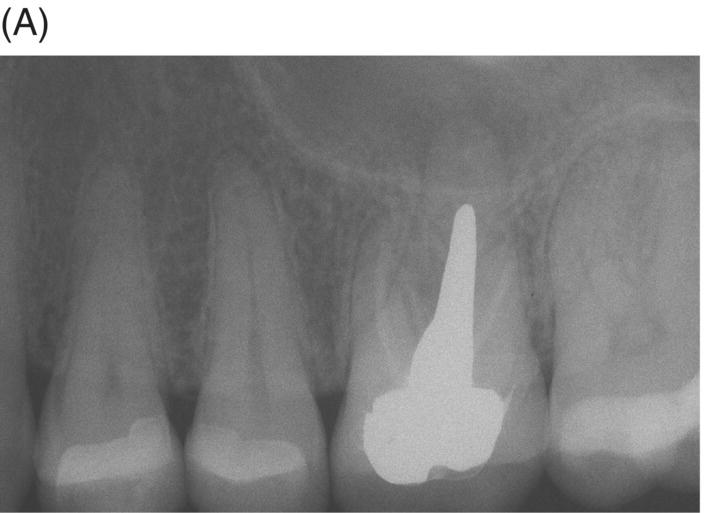
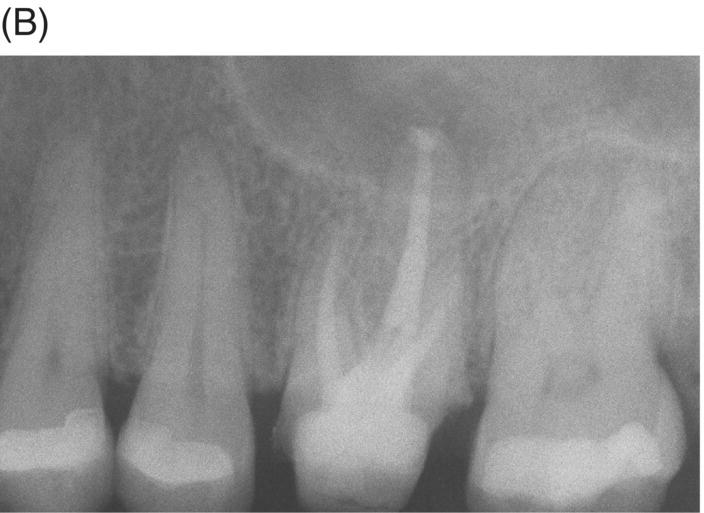
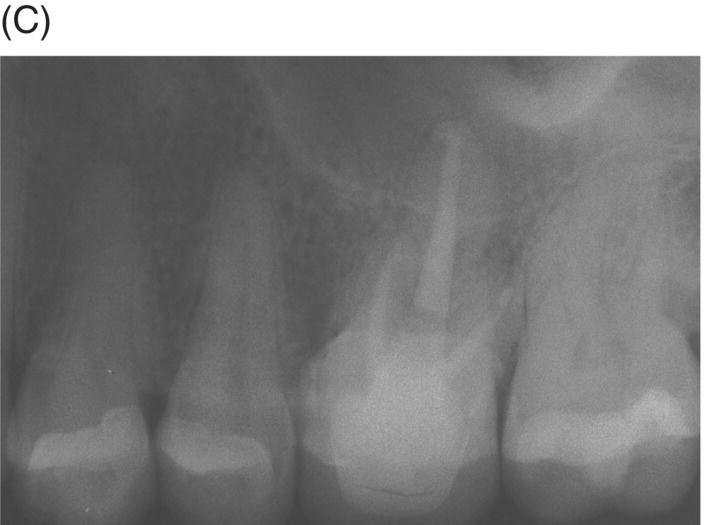
Fig. 8.3 (A) Radiograph of a symptomatic maxillary left first molar in a 38-year-old female featuring a large cast post and core and apical radiolucency with gutta-percha obturation and resorption at the palatal root. (B) Post-operative radiograph of MTA obturation of all canals, 5 mm apical fill at apex of palatal root. Canal was then backfilled with thermoplastic gutta-percha and a bonded core constructed. (C) Two-year 6 month review showing restoration with ceramic crown and complete periapical healing around palatal apex.
Retreatment
Retreatment of failed root canal treatments can be challenging when the original filling materials have sustained a long exposure to oral fluids. Bacteria in refractory or contaminated cases typically involve colonization of dentinal tubules by E. faecalis, C. albicans, and a host of Gram-positive bacteria, including Proprionibacterium, Actinomyces, Streptococcus and Peptostreptococcus species (Pinheiro et al. 2003; Siqueira & Rocas 2004; Williams et al. 2006). During long incubation periods, these micoorganisms have been known to advance and colonize dentinal tubules as far as 400 to 500 µm from the pulp–dentin interface (Orstavik & Haapasalo 1990; Peters et al. 2001; Love & Jenkinson 2002; Waltimo et al. 2003; Siqueira & Sen 2004). This characteristic can prevent their elimination even when long-term calcium hydroxide intracanal medication is used (Stuart et al. 2006). The eradication of these microbes is extremely difficult, even with the latest irrigants and technologies (Stuart et al. 2006). MTA obturation may impede the survival of these deeply imbedded microorganisms that might withstand conventional treatment (see Fig. 8.4).
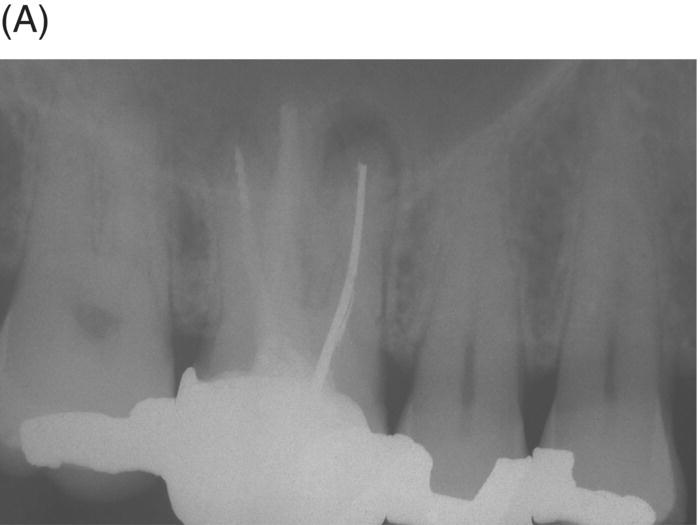
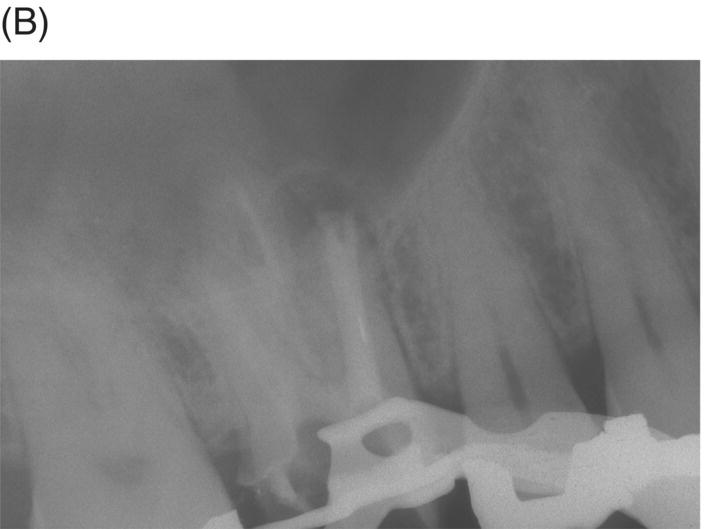
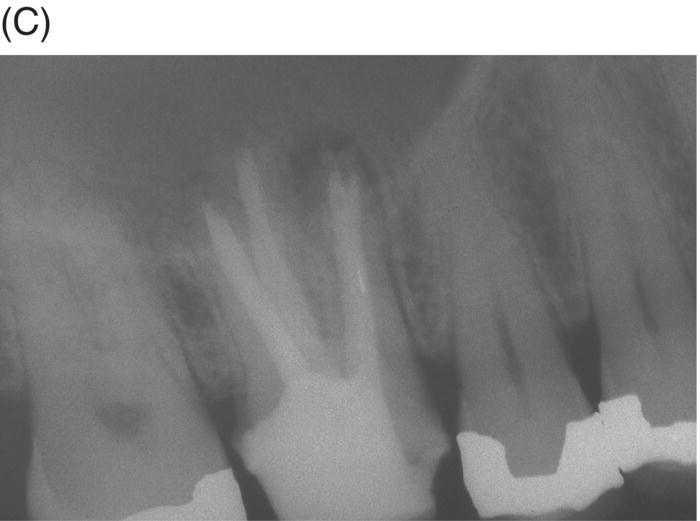
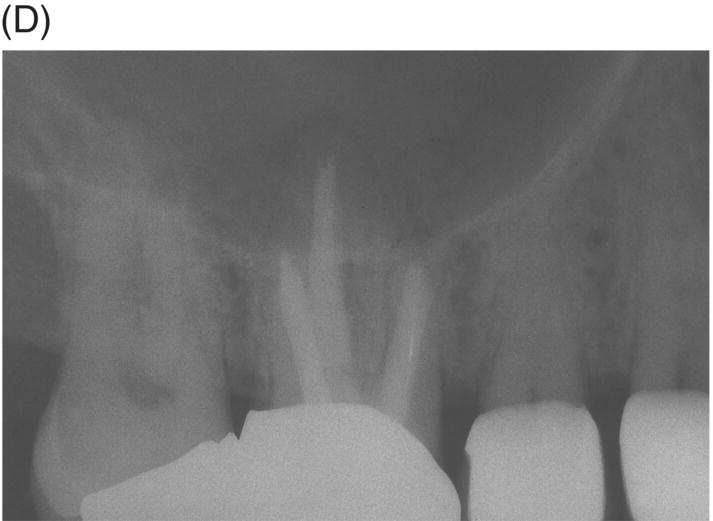
Fig. 8.4 (A) Forty-two year-old patient presented with his maxillary right first molar exhibiting ongoing symptoms and pathosis. Preoperative radiograph shows silver point obturation of mesiobuccal root associated with periapical pathosis and separated instrument at the apex of the distobuccal root. (B) Initial obturation of the MB root after silver point removal showing two portals of exit. (C) Complete obturation of three canals with MTA and placement of bonded core after MTA curing. (D) Eight-year recall showing absence of periapical pathosis. Patient was asymptomatic and the molar firm and in complete function.
A major problem with long-standing infections in previously treated teeth is the extent of bacterial invasion into the isthmuses, cul-de-sacs, accessory canals, and other anatomical ramifications (Nair et al. 2005; Ricucci & Siqueira 2010). These areas that were either not cleaned or are recolonized pose a challenge when using GP or resin-modified obturation materials due to their neutral pH and limited antimicrobial effect. Even if cleaning and shaping are completed with high regard for microscopic examination, ultrasonication and advanced irrigation systems using appropriate solutions, the elimination of these colonies may still not be adequate to avoid surgical indications. Long-standing colonization can also lead to biofilm formation within the canal system, which extends beyond the canal at the apex (Tronstad et al. 1990; Abou Rass & Bogen 1998; Sunde et al. 2000; Noguchi et al. 2005). These survival properties of polymicrobial colonies can prevent periradicular healing and may necessitate surgery to allow resolution of periapical disease when conventional filling materials are used. Healing seen with MTA retreatment can produce improved outcomes and appears to minimize the need for surgical intervention typically seen in some GP retreatments (see Fig. 8.5).
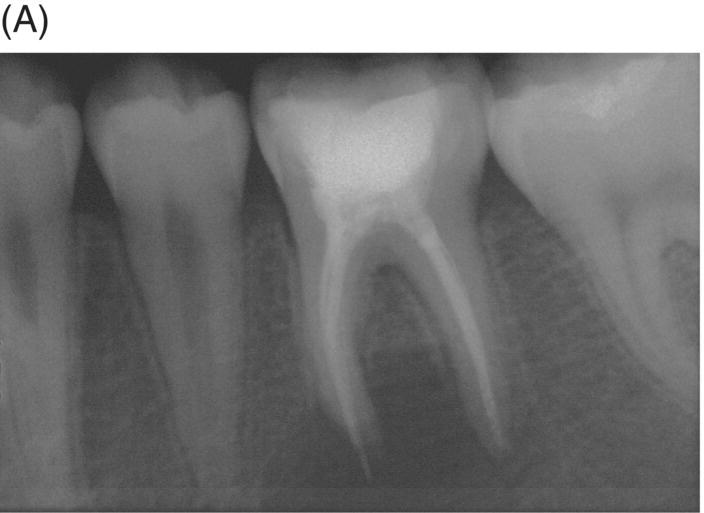
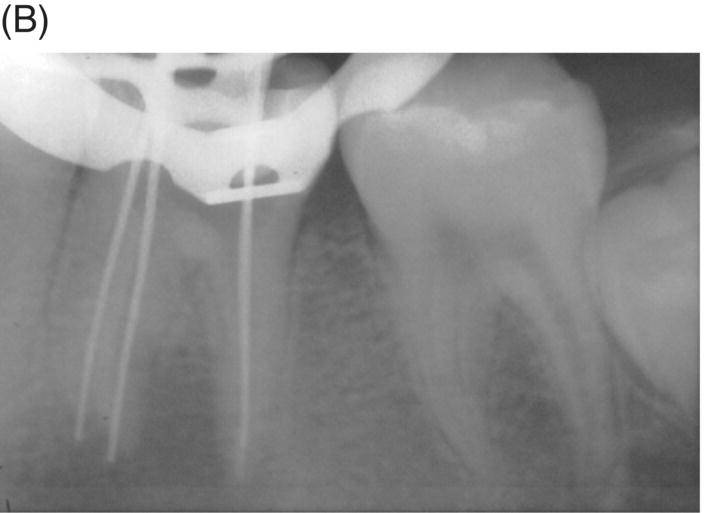
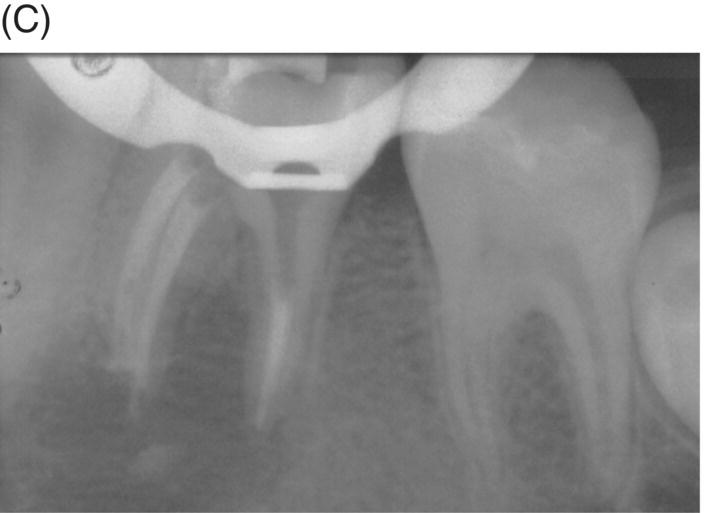
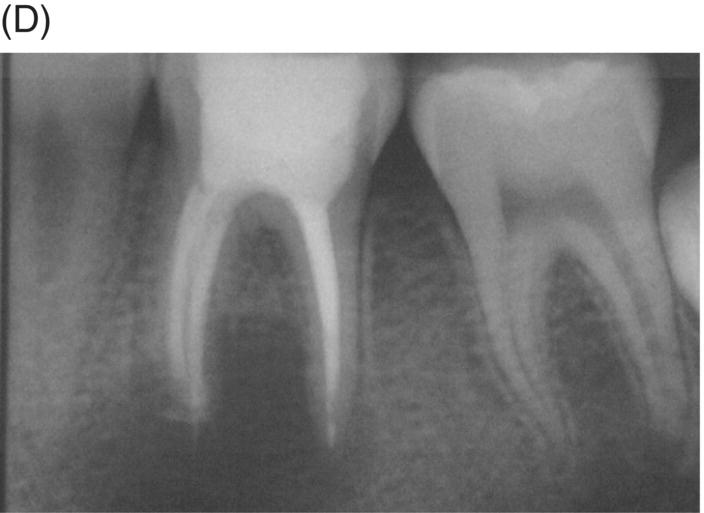
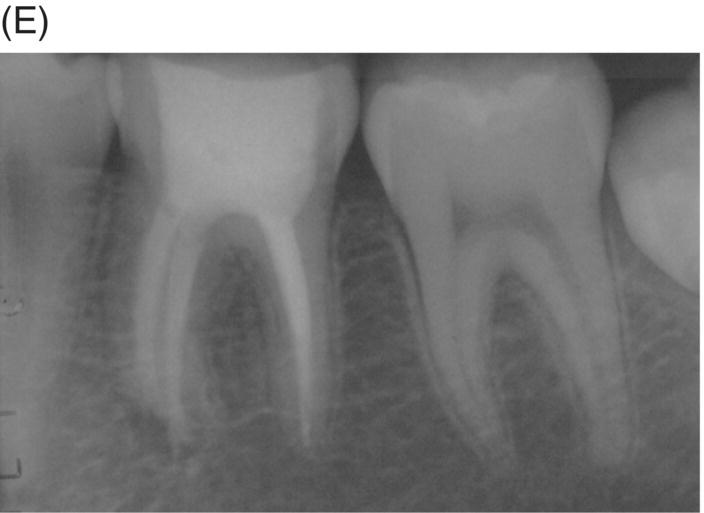
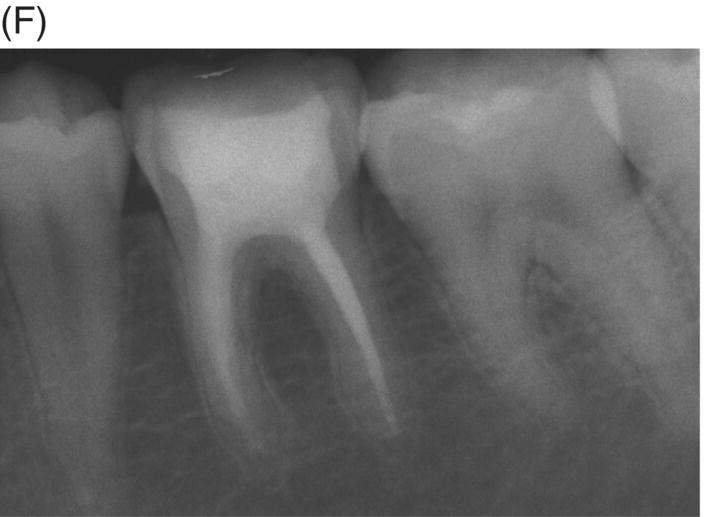
Fig. 8.5 (A) Periapical radiograph of a previously treated mandibular left first molar in a 12-year-old male patient with acute apical abscess and submandibular space infection. (B) Working length radiograph. (C) Initial MTA obturation with 5 mm apical plug to distal canal. (D) Radiograph of distal canal obturated with thermoplastic gutta-percha and sealer backfill. Bonded composite restoration is present. (E) Three-year 3 month radiographic review. (F) Nine-year 4 month radiographic recall without permanent cuspal coverage restoration. The molar exhibited normal probing and mobility.
Obturation prior to surgery
Obturation of teeth with MTA prior to surgical intervention allows clinicians to approach cases where anatomical access may be more than challenging. In particular, the mesial and distal roots of mandibular second and third molars and the palatal roots of maxillary premolars and molars may be considered. In other cases obturation of the distolingual root of mandibular first and second molars prior to surgery can simplifiy management of the resected root. After MTA has cured, resection does not affect seal integrity and encourages complete healing after removal of infected tissues (Andelin et al. 2002; Lamb et al. 2003). This surgical technique also allows a more conservative osteotomy site as the remaining root may not require enhanced access for the introduction of micromirrors, ultrasonic tips and materials. Nevertheless, the presence of GP remaining against the canal wall after resection mandates retrofill placement. A root-end filling material should also be considered if plastic or metal GP carriers are present.
Gutta-percha remaining at the canal interface poses a dilemma since tenacious Gram-positive bacteria and fungi show an ability to survive between GP/sealer and dentin (Friedman 2008). This aspect of MTA retreatment and surgical execution is an important consideration since previous treatments can present with transportation, pulpal remnants, voids, sealer absence, accessory canals, untreated isthmuses, separated instruments and biofilms, all of which can delay or prevent healing. Placement of a root-end filling material after root resection of canals filled with MTA should be considered when radiographs or microscopic examination raise concern (see Figs 8.6 and 8.7).
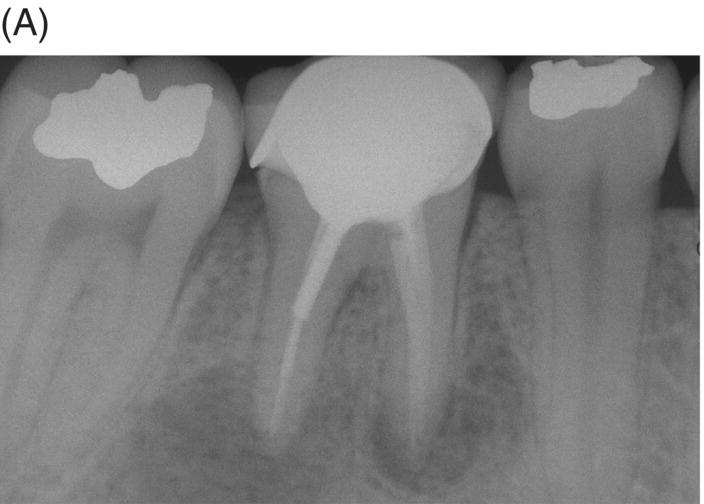
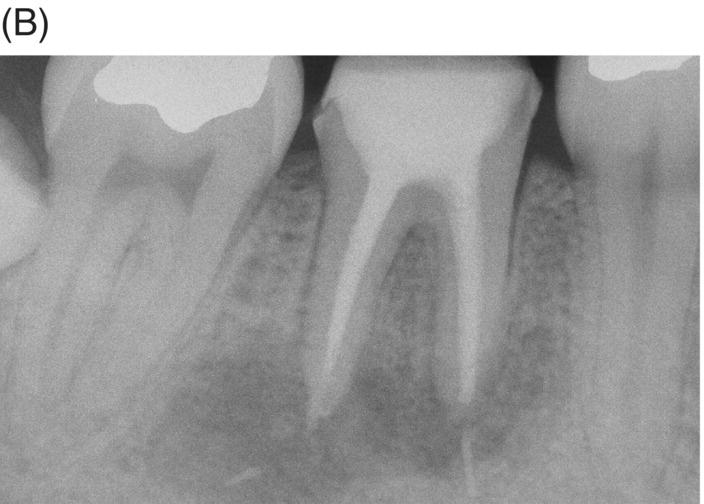
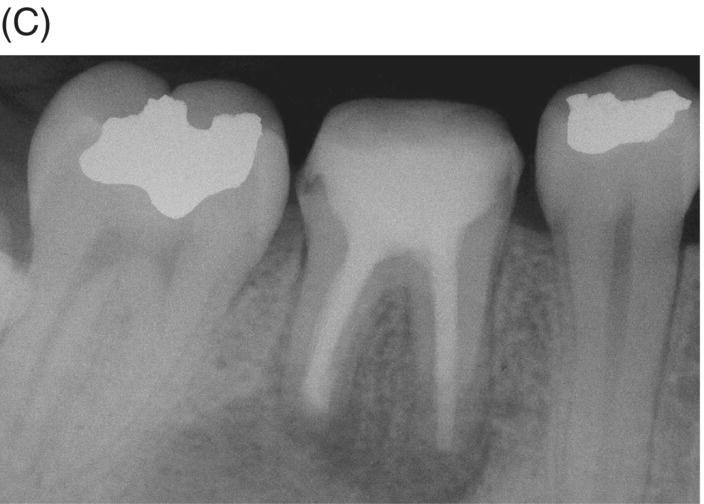
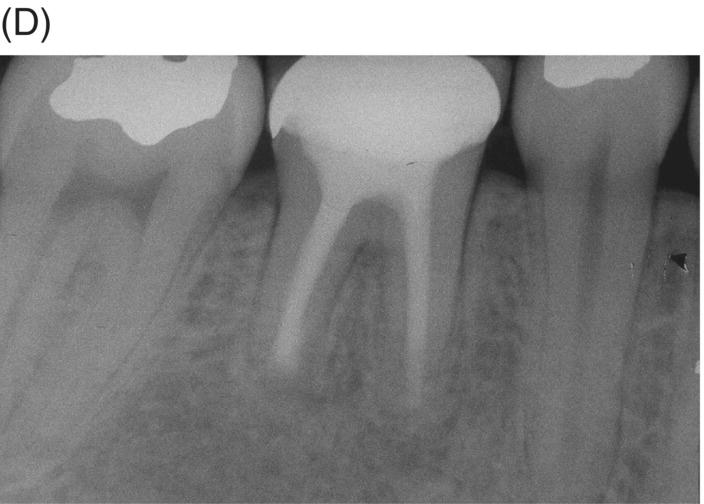
Fig. 8.6 (A) Radiograph of previously treated symptomatic mandibular right first molar with extensive apical pathosis and submandibular space infection in a 26-year old female. (B) Postoperative radiograph after disassembly, gutta-percha removal, MTA obturation and bonded core placement. Note extruded gutta-percha after retreatment. (C) Surgical root resection with removal of extruded gutta-percha and inflammatory tissue. Microscopic examination after resection revealed remaining gutta-percha located in distal canal wall interface and MTA retrofill was placed. (D) One year 6-month recall radiograph shows new full coverage restoration and complete remineralization of the previous osteotomy site.
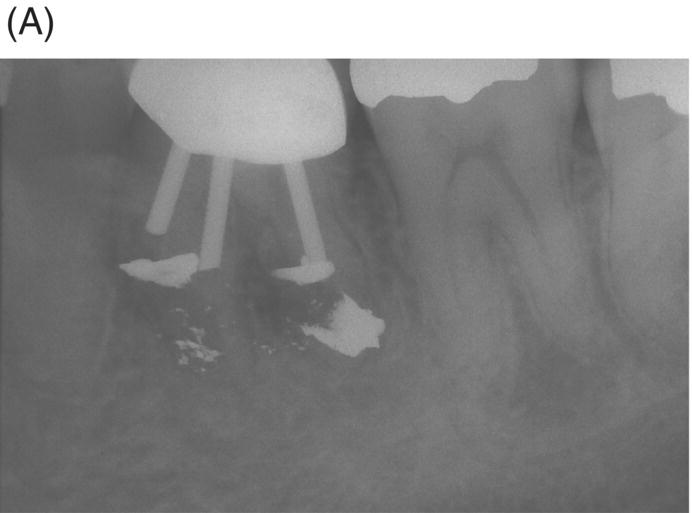
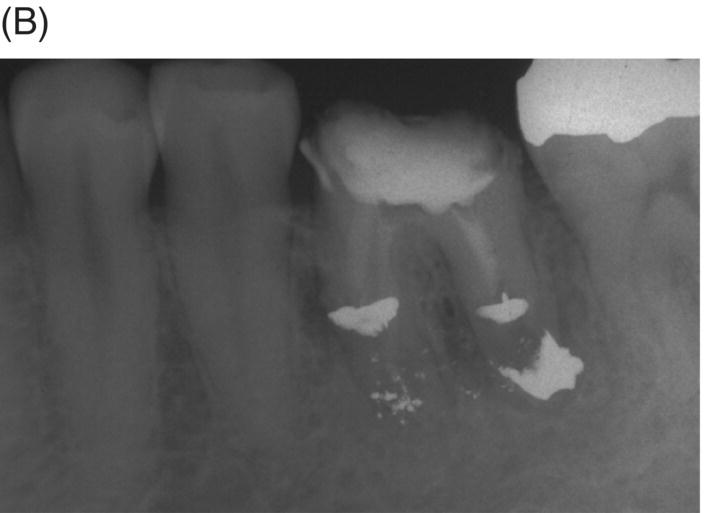
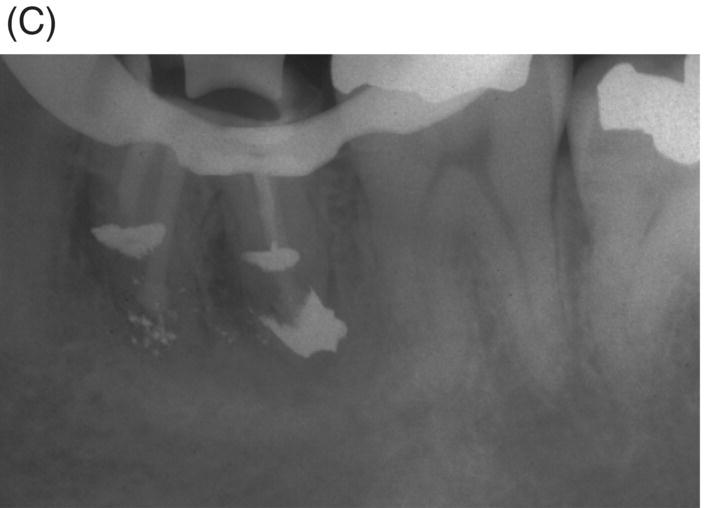
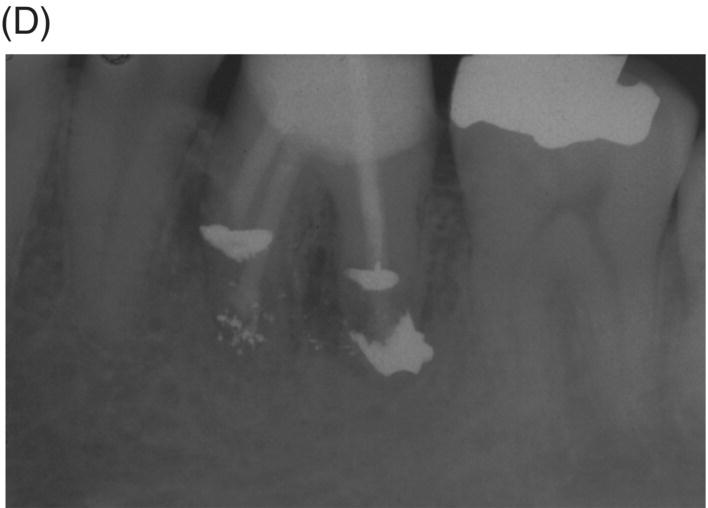
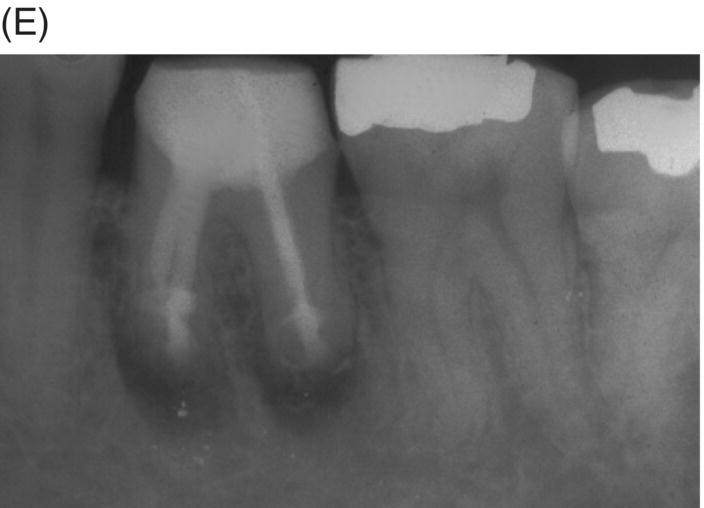
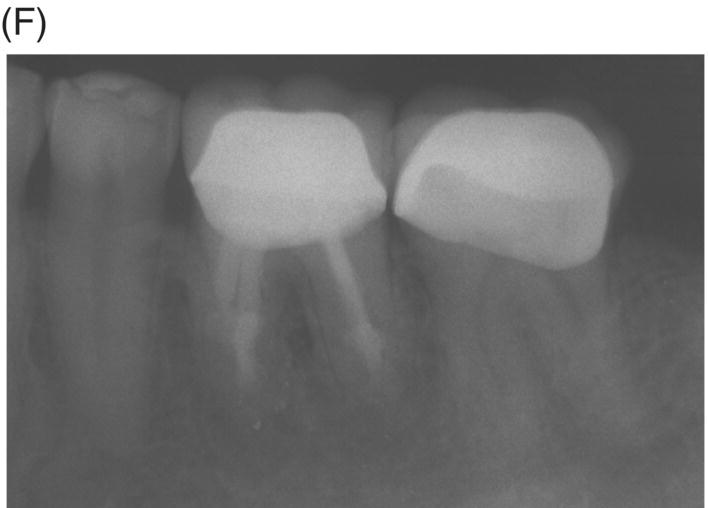
Fig. 8.7 (A) Thirty-four year-old male patient with symptomatic mandibular left first molar with three metal posts. Previously treated surgically with amalgam retrofills and treatment planned for extraction and an implant. (B) Calcium hydroxide dressing after crown and post disassembly. (C) All canals obturated with MTA. (D) Post and core constructed after MTA curing. (E) Postoperative radiograph after second surgery, removal of amalgam retrofills and MTA root-end restorations. (F) Eight-year 5 month recall showing remineralization of osteotomy site. The patient was symptom-free.
Large and long-standing lesions (over 5 mm) can show extensive bone loss leading some clinicians to condemn these treatment failures to extraction and implant placement (Greenstein et al. 2008). MTA obturation in retreatment combined with surgery can now provide patients an opportunity to retain teeth that have been compromised by advanced and extensive pathosis (see Fig. 8.8).
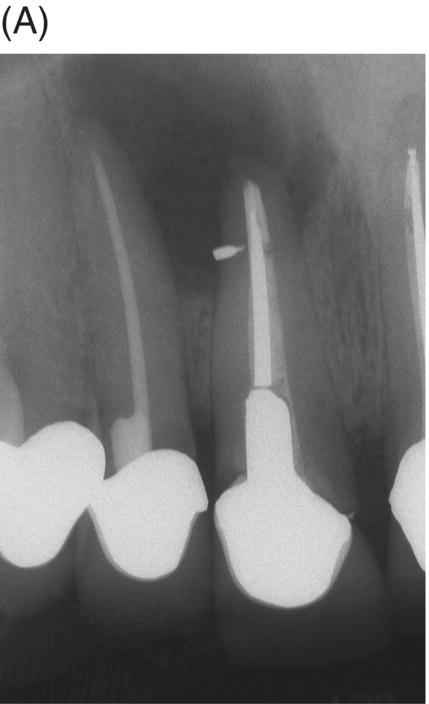
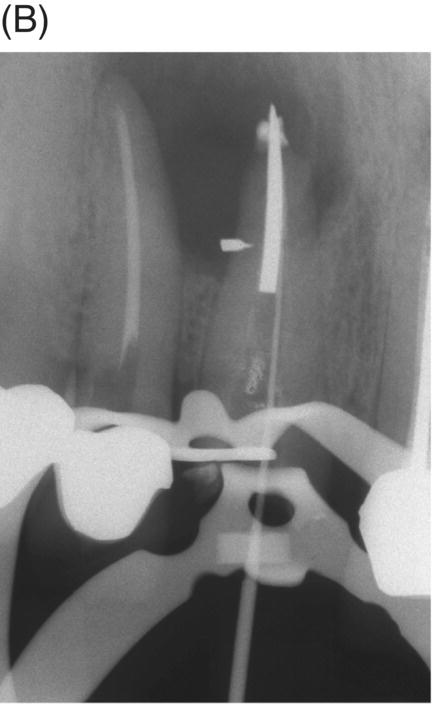
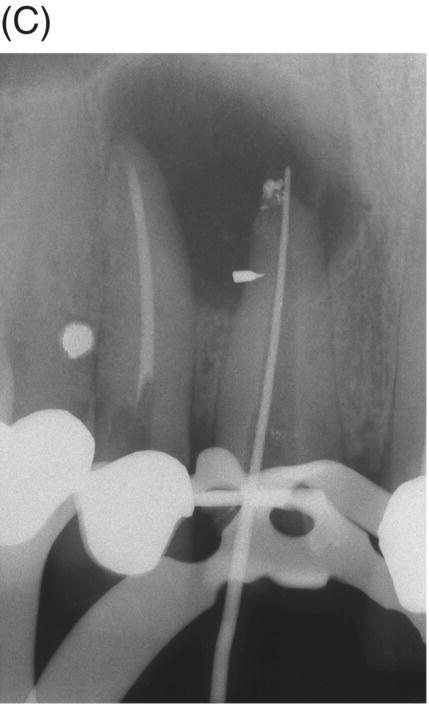
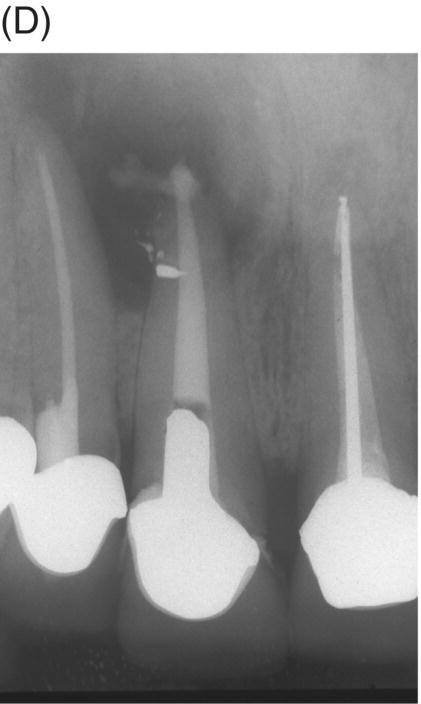
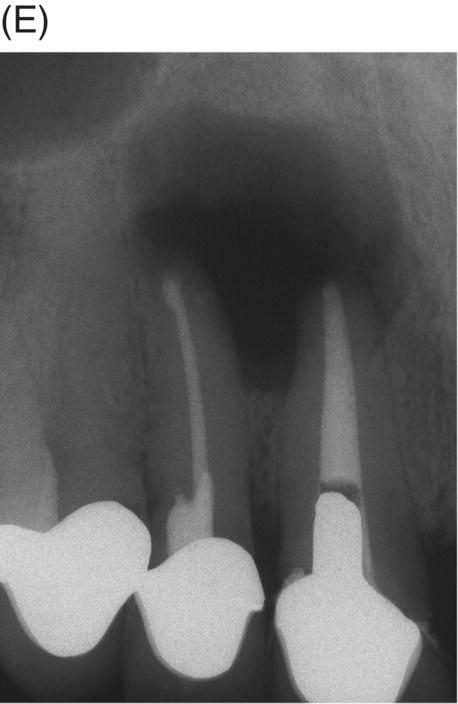
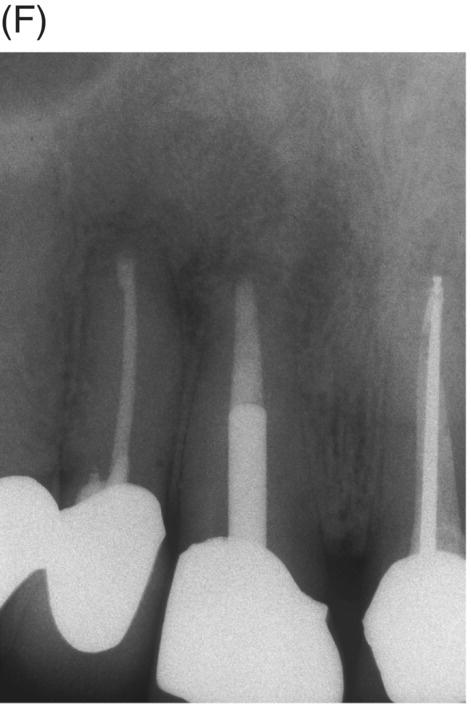
Fig. 8.8 (A) Preoperative radiograph of failed silver point treatment. Sinus tract formation and a large periapical lesion associated with the maxillary right central incisor extending to the right lateral incisor. (B) Attempted bypass of silver point during orthograde retreatment. (C) Radiograph after silver point removal and extrusion of canal materials. (D) MTA obturation prior to provisional cementation of the disassembled cast post and core. (E) Root resection of both the central and lateral incisors and removal of extruded material and silver point debris. (F) Four-year 6 month radiographic recall showing normal periapical structures and alveolar bone. Courtesy of Dr. Laureen M. Roh, Los Angeles, California.
Obturation with perforation repair
Perforations are iatrogenic or pathologic communications between the root canal and the periodontium. MTA has been shown to be the preferred material in managing this complication (Main et al. 2004; Pace et al. 2008). Repair using MTA involves the identification of the involved area and application of the cement in a thickness that provides an adequate seal to promote repair and healing. This procedure can be performed nonsurgically or surgically using a dental operating microscope (DOM). However, perforations do not always occur on the pulpal floor or accessible areas that allow for simple repair and further visits follow, often with GP and sealer obturations. The possibility of dislodging the MTA when placing the second obturation material is a strong argument for obturation of the entire canal with MTA when the perforation site is below the canal orifice.
There is no documented advantage to repairing a radicular perforation followed by GP and sealer obturation. The most logical sequence involves obturating the entire canal, unless a post space is to be prepared which may complicate treatment options. Obturation of the entire canal in resorption and perforation cases can be more predictable owing to MTA thickness and overall cement stability (Tsai et al. 2006). If the canal is treatment planned for post placement, then the MTA repair must be customized to accommodate the indicated restorative requirements. Clinicians should consider the simplicity of using only one material to fill the entire canal to the orifice when these defects are present (see Fig. 8.9).
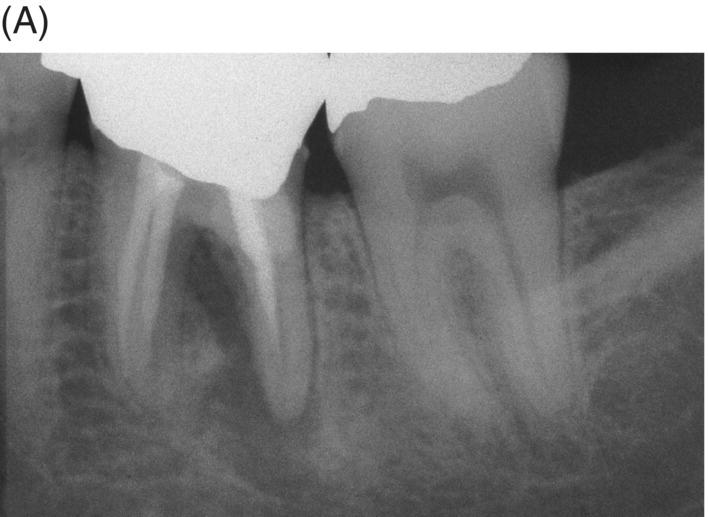
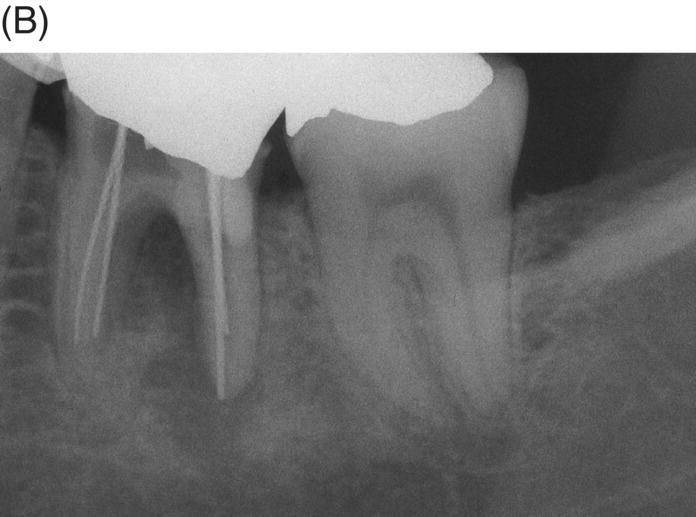
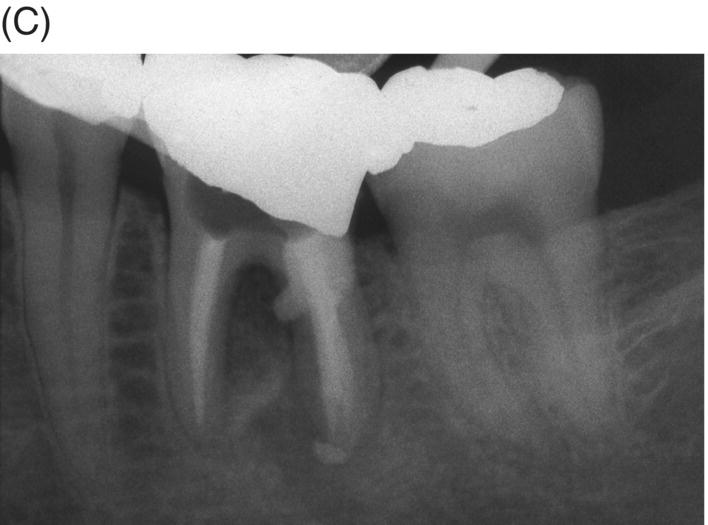
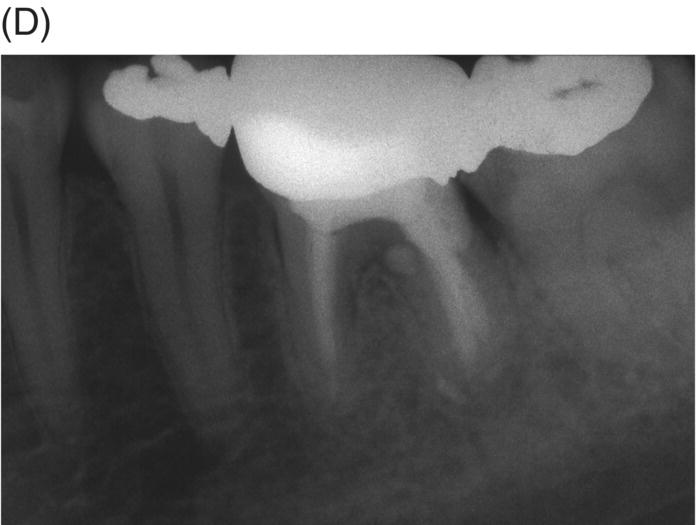
Fig. 8.9 (A) Preoperative radiograph of a failed gutta-percha obturation on the left first mandibular molar in a 67-year-old patient. Note presence of furcation bone loss. (B) Working length radiograph; apical resorption at distal apex. (C) Initial obturation of all canals with gray MTA revealing extrusion of material through perforating resorptive defect at furcation. (D) Eighteen month recall showing complete osseous repair of furca and periapical area.
Apexification using MTA obturation
The induction of root-end closure by MTA is a predictable and desired goal for treating nonvital, infected immature permanent teeth (Shabahang & Torabinejad 2000; Giuliani et al. 2002; Witherspoon et al. 2008; Pace et al. 2008; Bogen & Chandler 2008; Nayar et al. 2009; Güneş et al. 2012). If regenerative endodontic procedures are not elected (see Chapter 6), then the formation of a root-end plug using MTA can provide an option that encourages root maturation. MTA stimulates cementogenesis and odontogenesis, allowing the cells of the apical papilla to complete root formation and apical closure (Huang et al. 2008). This process is completed by the cells of the apical papilla that differentiate into cementoblasts and odontoblasts (see Fig. 8.10).
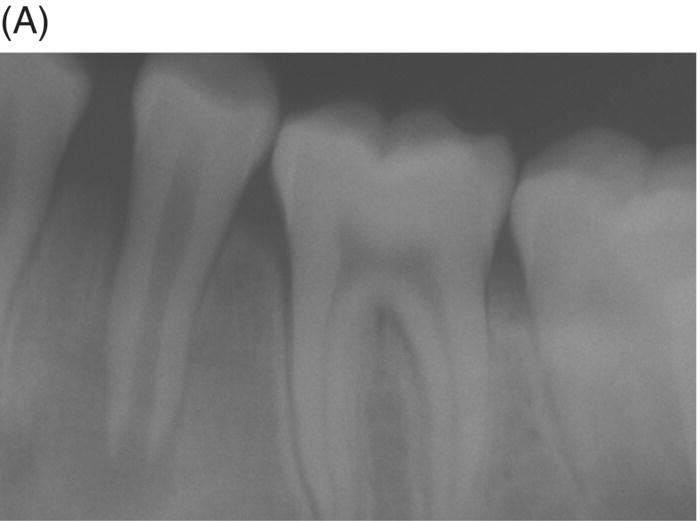
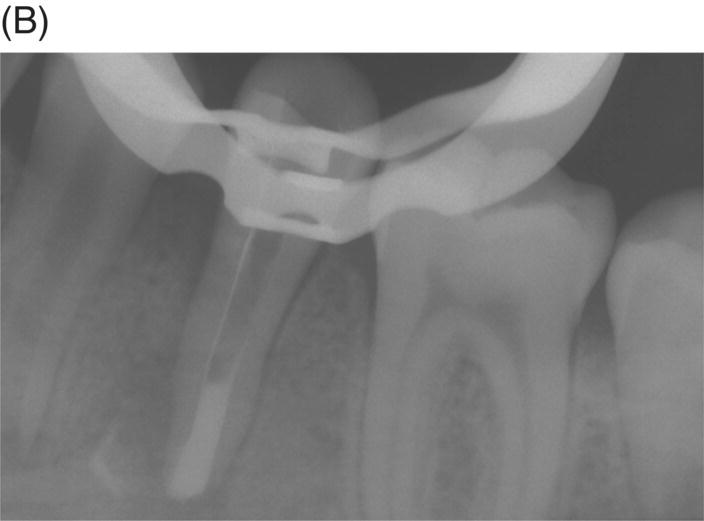
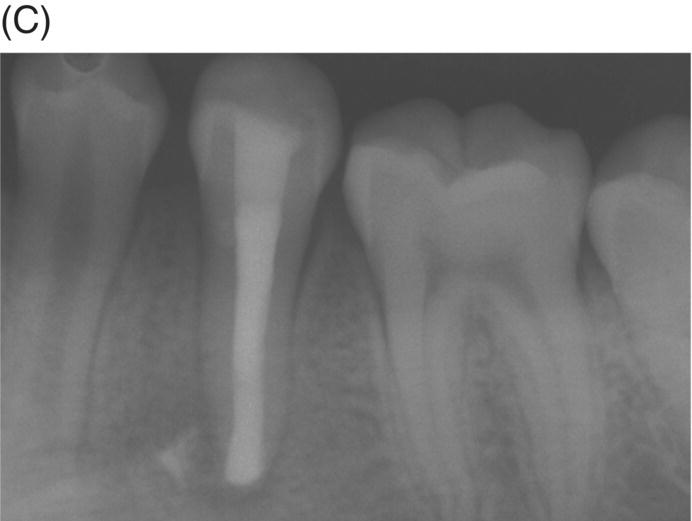
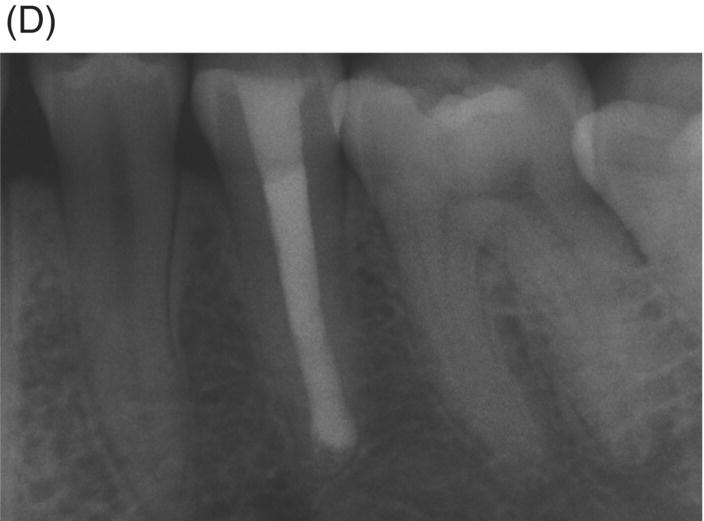
Fig. 8.10 (A) A 10 year-old patient with a symptomatic mandibular left second premolar with dens evaginatus and open apex with extensive periapical disease. (B) Obturation of apical foramen with 5.0 mm gray MTA apical plug. (C) Final obturation with thermoplastic gutta-percha and a bonded restoration. (D) Nine-year radiographic review. Note mature apex formation.
The obturation technique in teeth with open apices is completed after proper cleaning and shaping to the working length. Length is usually determined by estimation using pretreatment images and confirmed using a large master apical file. The aim during careful instrumentation is to clean the canal walls to the limit of the apical extension, protecting and preserving the tissue beyond the root apex. Tissues beyond or at the apex will act as a natural barrier or diaphragm during the obturation process. Apical obturation must be completed using gentle force and with extreme tactile care. The obturation should ideally be positioned 1 mm short or level with the apical rim or collar (see Chapter 5).
Compaction can be completed with a master apical file (MAF) one or two sizes smaller, endodontic pluggers, a Glick instrument, a large GP master cone, or the back end of a coarse paper point. Ultrasonic tips can be used, but care must be given to use the device at a low setting to avoid forcing an excess of MTA beyond the apex. Although MTA extrusion routin/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


