5
Management of Teeth with Necrotic Pulps and Open Apices
Shahrokh Shabahang1 and David E. Witherspoon2
1 Department of Endodontics, Loma Linda University School of Dentistry, USA
2 North Texas Endodontic Associates, USA
DIAGNOSIS IN IMMATURE TEETH
Development of the dental root structure requires a vital pulp. If the pulp is vital and the apex is not formed, it is imperative to take the necessary steps to maintain the vitality of the pulp. These measures promote the apex to complete its formation and calcification since it is only pulpal tissue that has the ability to form true dentin (Goldman 1974). Disruption of pulp vitality will interfere with continued root development. Ideally, where possible, pulp vitality must be maintained in immature permanent teeth to allow completion of root development. The pulp tissues should be removed only when irreversibly inflamed or necrotic. Typically, the dental pulp tissue in young patients has been exposed to less irritation, is more cellular and therefore better able to recover from injury. Cvek and coworkers (Cvek et al. 1982) demonstrated that complex crown fractured teeth show vital pulp tissue after being exposed for up to seven days post injury, with only 2 mm of the pulp beneath the exposure level exhibiting inflammation.
Clearly, proper assessment of the pulpal status to allow an accurate diagnosis is critical prior to determining the treatment plan for teeth with underdeveloped roots. Before determination of the best treatment option, pulp vitality must be assessed. If vital, every effort must be made to maintain its vitality to allow continued root development. Figure 5.1 presents a flow chart to facilitate the decision-making process when treating permanent teeth with incompletely developed roots.
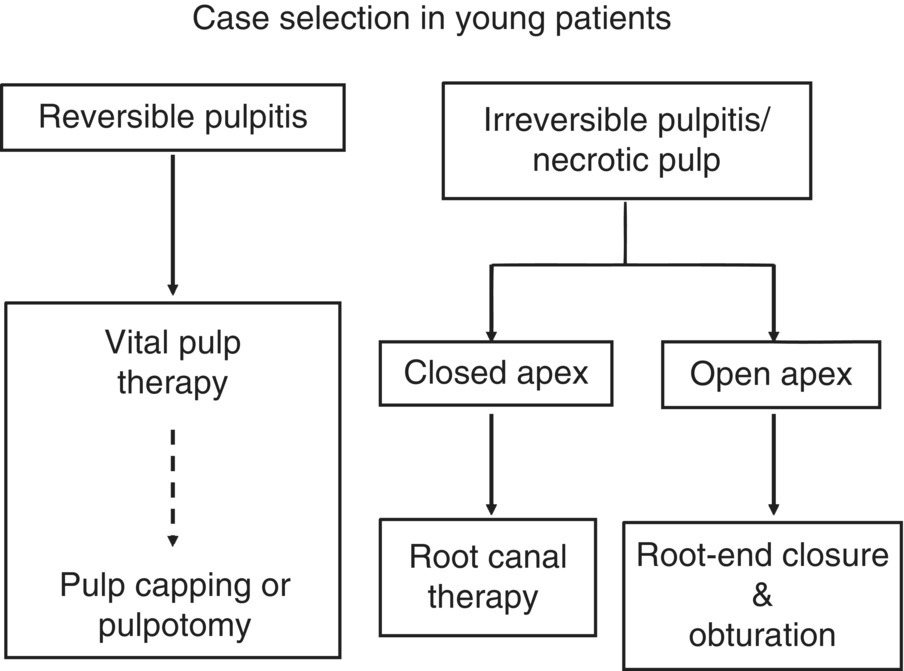
Fig. 5.1 Case selection for treatment of permanent teeth with incomplete root development.
Assessment of the tooth in question is made using radiographic evaluation to determine the maturity of the developing root and clinical evaluation based on history and clinical testing. immature teeth are usually encountered in children. Pulp testing in children is a complex procedure and subjective in nature. It is impacted by their linguistic development and expectations (Pinkham 1997; Toole et al. 2000; Harman et al. 2005). The less linguistically developed and the higher their expectations of a negative experience, the less discriminating their response might be (Toole et al. 2000; Harman et al. 2005). Diagnosis can be confused by the patient’s subjective symptomatology, which may not correctly echo the real histological status of the pulp (Camp 2008). The response to pulp testing is also less predictable than in adults, leading to a greater possibility of false negatives. Generally, the response to cold stimulus produces the most reliable result (Fulling & Andreasen 1976; Fuss et al. 1986). Electric pulp testing in teeth with open immature apices can produce a high percentage of false negatives (Klein 1978). The history is also important not only to determine any predisposing conditions, which may impact pulpal healing, but also symptoms and presence of certain complicating factors such as traumatic injuries to the tooth. For instance, hyperglycemia may significantly impact pulpal healing (Garber et al. 2009). Using laser doppler measurement of pulpal blood flow (PBF), Strobl and coworkers (2004) were able to show that lateral and extrusive subluxation injuries do not significantly impact PBF. On the other hand, intrusive injuries lead to diminished PBF and pulp necrosis (Fig. 5.2). Additionally, determination of pulp status may be accomplished by direct viewing of the pulpal tissues if it is exposed. Diagnosing reversible pulpitis can be most reliably established by assessing the clinical ability to achieve hemostasis using NaOCl for 5 to 10 min after the pulp has been clinically exposed (Matsuo et al. 1996; Bogen et al. 2008).
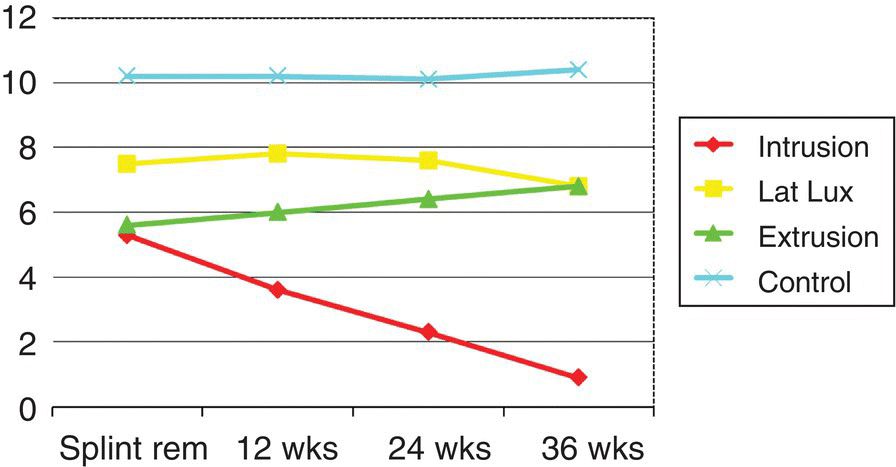
Fig. 5.2 Pulpal blood flow measurement using laser Doppler shows negative impact of intrusive injuries.
A comprehensive understanding of the radiographic appearance of normal root formation is indispensable in correctly diagnosing periradicular pathosis associated with immature teeth. In developing stages, the buccal–lingual dimension of the root canal of incisors is typically greater than the mesial–distal dimension. A root canal with parallel walls mesiodistally is inclined to have divergent walls and greater width labio-lingually; a canal with tapering walls mesiodistally tends to have parallel walls and greater width labio-lingually. The lag in root development usually exists for more than three years after eruption of the tooth (Duell 1973). This developmental pattern can be misleading with respect to convergence and divergence. Since the buccal–lingual aspect of the root canal is the last to become convergent as the root develops, it is possible to have a radiograph showing an apically convergent root canal, which in the buccal-lingual plane is divergent (Camp 1980). There are factors aside from the apical anatomy that can be misleading. The tissue forming apically may appear radiographically complete, but is often porous as the developing root diaphragm in the labiolingual plane lags behind that in the mesio-distal plane of the immature root (Gutmann & Heaton 1981). However, with the advent of cone beam computerized tomography, the difficulty in assessing apical formation is minimized (Patel 2010).
As Fig. 5.1 shows, if the pulp is deemed necrotic, root canal therapy is indicated; however, because of the presence of an open apex, root end closure must be achieved prior to completion of root canal therapy.
HISTORY OF TREATING IMMATURE TEETH
Traditionally, the treatment of immature teeth has been challenging. Typically, these teeth inherently have several treatment challenges that are not encountered when teeth in adult patients. The apical diameter of the canal is often larger than the coronal diameter (Friend 1969), rendering mechanical root canal debridement difficult. The lack of an apical constriction makes canal obturation in all dimensions difficult. The thin walls of the root canal are prone to fracture, rendering surgical intervention and non-surgical root canal therapy techniques that require significant compaction during obturation as undesirable options. Historically, techniques for the management of non-vital immature teeth have included custom fitting gutta-percha cones as the filling material without a prior apexification procedure (Stewart 1963; Friend 1966), paste fills (Friend 1967), apical surgery (Ingle 1965), and often extraction (Rule & Winter 1966).
In 1940, Rohner (1940) described the use of a Calxyl paste to be placed over the pulp remnant after vital pulpectomy. The result was formation of an apical barrier. The first description of the use of calcium hydroxide (CH) as an agent to induce apical closure was in 1953 (Marmasse 1953). This was followed by Granath in 1959 (Granath 1959); subsequently in 1962 Matsumiya (Matsumiya et al. 1962) and in 1964 Kaiser (Kaiser 1964) also reported on the use of CH in apical closure procedures. At this time several other materials were also being utilized for this procedure; these included Tricresol and formalin (Cooke & Rowbotham 1960) and antibiotic pastes (Herbert 1959; Ball 1964). The term apexification was used to describe the procedure for inducing apical closure to facilitate obturation of the root canal system and was popularized in the 1960s (Frank 1966; Steiner et al. 1968). It thus became the treatment protocol of choice for immature necrotic teeth. In addition to the use of CH, various other procedures and medicaments have been recommended for inducing apexification with varying degrees of success. These include inducing a blood clot (Ham et al. 1972), and placement of materials such as Tricresol and formalin (Cooke & Rowbotham 1960), antibiotic pastes (Ball 1964), tricalcium phosphate (Koenigs et al. 1975; Roberts & Brilliant 1975), collagen–calcium phosphate gel (Nevins et al. 1977, 1978; Citrome et al. 1979), CH in different mixes such as camphorated parachlorophenol (CMCP) (Frank 1966; Dylewski 1971; Steiner & Van Hassel 1971; Ham et al. 1972; Torneck et al. 1973), iodoform (Holland et al. 1973), water (Binnie & Rowe 1973; Wechsler et al. 1978), local anesthetic solution, isotonic saline solution, glycerol, and others (Heithersay 1970; Vojinovic & Srnie 1975; Camp 1980; Webber et al. 1981). Several case reports have also demonstrated continued apical development in non-vital teeth after removal of the non-vital tissue and control of infection alone (Das 1980; Cameron 1986). Additionally, Lieberman and Trowbridge (1983) reported a case in which apical closures of non-vital permanent incisors were observed without any treatment at all.
Regardless of the many approaches to apexification, CH has become the material of choice for this procedure since the 1960s. This is mainly due to Frank’s (1966) landmark paper where he proposed the use of a thick paste of CH and CMCP. After placement of the paste, the tooth would require evaluations every 3–6 months until closure of the apex as determined radiographically or clinically (manifested by a hard barrier at the root-end detected with an endodontic instrument). He further proposed that, with this procedure, the clinical results might vary and present as follows: (1) an apex that closes with a definite, though minimal, recession of the root canal (the apical aspect continues to develop with a seemingly obliterated apex); (2) the obliterated apex may develop without any change in the root canal space; (3) there may be no radiographic evidence of any development in the periapex or root canal, however, an instrument inserted into the canal encounters a definite stop that would allow for the filling procedure; or (4) a calcific bridge may form just coronal to the apex, which can be determined radiographically. Feiglin (1985), similarly classified various categories of apex formation following apexification procedures with CH and listed them as follows: (1) The apex bridged over with hard tissue that would presumably be osteocementum or osteodentin; (2) a perfectly normal apex formed from the point of trauma to the apex of the tooth; (3–4) variable amounts of vital tissue remained above the point of trauma, which allowed the root canal to heal or bridge over and the apex to continue forming and to calcify; and (5) apex lifted off during tooth movement.
INFECTION CONTROL IN IMMATURE TEETH
Immature permanent teeth pose special challenges during endodontic procedures not only because of the wide-open root apex, but also because of the thin dentin walls (Fig. 5.3). As such, debridement is completed primarily by chemical means to remove any remaining pulp tissues and to disinfect the canal system. Furthermore, an accurate determination of root length is required to ensure complete canal debridement and to confine treatment materials to the canal space. This would prevent damaging of the very valuable remnants of the Hertwig’s epithelial root sheath (HERS) (Fig. 5.4).
Fig. 5.3 Radiographic view of an immature permanent incisor with open apex and thin dentin walls.
Fig. 5.4 Hertwig’s epithelial root sheath with BMP-2 immunoreactivity stain.
Generally, electronic apex locators are not accurate in teeth with wide open apices (Hulsmann and Pieper 1989). Radiographic root determination is the best means to obtain accurate root length measurement (Fig. 5.5).
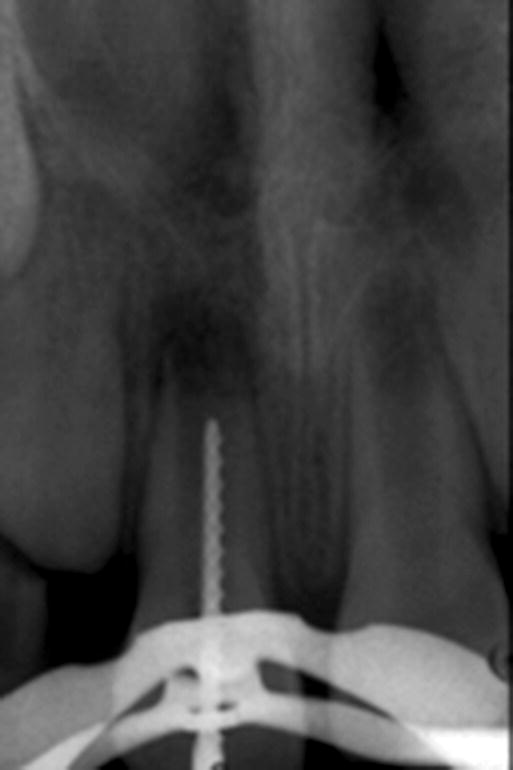
Fig. 5.5 Demonstration of radiographic root length determination in an incisor with a wide-open apical foramen.
Sodium hypochlorite (NaOCl) and CH have good tissue dissolving properties as well as antimicrobial activity (The 1979; Cunningham & Balekjian 1980; Cunningham & Joseph 1980; Morgan et al. 1991; Baumgartner & Cuenin 1992; Yang et al. 1995; Turkun & Cengiz 1997; Wadachi et al. 1998; Gomes et al. 2001). While NaOCl exerts its effect during the course of the procedure, CH requires additional exposure time. A 1-week obturation of the canal space with CH will allow disinfection along with dissolution and removal of pulpal remnants (Sjogren et al. 1991; Turkun & Cengiz 1997). Despite its convenient availability, several investigators have reported shortcomings with respect to NaOCl’s ability to completely disinfect the root canal space (Shabahang et al. 2003; Waltimo et al. 2005; Siqueira & Rocas 2008). Inconsistencies in reports may be due to differences in methodologies or the actual usage protocols. In fact, NaOCl is most effective when mixed into solution just before use (Johnson & Remeikis 1993). Heat, light and exposure to oxygen may readily inactivate NaOCl (Gerhardt & Williams 1991; Clarkson et al. 2001).
Long-term exposure to CH may also have detrimental effects on dentin. Studies have shown that long-term CH therapy that would expose root dentin to CH for periods exceeding one month results in structural changes in dentin that yield significantly higher susceptibility to root fracture (Andreasen et al. 2002, 2006; White et al. 2002; Doyon et al. 2005; Rosenberg et al. 2007; Hatibovic-Kofman et al. 2008; Tuna et al. 2011; Bakland and Andreasen 2012). Previous reports of failures of teeth with a history of apexification due to cervical root fractures (Cvek 1992) may be attributed, not only to the thin dentin walls, but also to excessive exposure of the dentin to CH.
In recent years, several investigative groups have revisited antibiotic agents for root canal disinfection. Use of antibiotics is not a new approach to root canal disinfection. In 1980, Das (1980) reported successful apexification of a tooth after root canal debridement and intracanal antibiotic therapy with an oxytetracycline HCl ointment. During the past decade, addition of antibiotic products to debridement protocols has regained popularity.
In 2003, Torabinejad and his group published a series of reports to demonstrate the advantages of a mixture of doxycycline, citric acid, and detergent (BioPure MTAD) over NaOCl and other commonly used root canal irrigants (Beltz et al. 2003; Shabahang et al. 2003; Shabahang & Torabinejad 2003; Torabinejad et al. 2003a, b, c). BioPure MTAD is effective as a final rinse prior to obturation to disinfect the root canal system and to remove the smear layer.
In 2001, Iwaya and associates (2001) published a case that demonstrated the effectiveness of a double antibiotic paste to disinfect a premolar with a necrotic pulp and a large periradicular radiolucent lesion; subsequently Banchs and Trope (2004) published a similar case. In this case report and subsequent studies, Trope’s group recommended a mixture of metronidazole, ciprofloxacin, and minocycline. In in-vitro studies, this antibiotic combination has shown potential to disinfect the root canal system (Hoshino et al. 1996; Sato et al. 1996). In an animal experiment, significantly better disinfection was achieved after dressing with the triple antibiotic paste compared with biomechanical debridement using 1.25% NaOCl (Windley et al. 2005). Due to potential staining caused by longer exposures to minocycline (Kim et al. 2010; Nosrat et al. 2012), some investigators have opted to omit the minocycline and use a double antibiotic mix.
APEXIFICATION
If pulpal necrosis occurs in immature teeth, an alternative treatment approach to conventional root canal therapy must be employed due to the presence of an open apex. The young pulpless tooth, frequently, has thin, fragile walls making it difficult to adequately clean and to obtain the necessary apical seal (Frank 1966). Traditionally, the approach has been to utilize CH to induce apexification following disinfection of the root canals in the conventional manner (Seltzer 1988). Completion of endodontic therapy has been typically delayed until root-end closure has been completed through apexification. Apexification is defined as “a method of inducing a calcified barrier in a root with an open apex or the continued apical development of an incompletely formed root in teeth with a necrotic pulp” (Anonymous 2003). Apexification employing CH in one form or another has a long history in the treatment of non-vital immature teeth.
CALCIUM HYDROXIDE APEXIFICATION THERAPY: OUTCOMES
A number of studies have examined apexification outcomes both in terms of success and length of time it takes to achieve apical closure using CH. In 1970, Heithersay (1970) reported on 21 clinical cases, which were treated with CH in a methylcellulose carrier (Pulpdent) over an observation period of 14–75 months. The results showed that in teeth with continued apical development, a definite fine apical canal could be seen radiographically, but there was no definite apical barrier clinically. In their 1987 report, Ghose and associates (1987) used Calasept in traumatized, partially developed permanent central incisors of 43 patients between the ages of 8 and 12 years. All of the teeth had crown fractures with pulp exposures. The pulps had been exposed to the oral environment for one month to three years and all were necrotic. Forty-nine of 51 teeth developed an apical barrier that could be confirmed clinically and radiographically. The time needed to form an apical barrier of hard tissue ranged from 3–10 months. Morfis and Siskos (1991) reported on the outcomes of 34 cases in which apexification was performed using CH. In all cases, chemically pure CH powder was mixed with anesthetic solution. Twelve patients were aged 27–40 years and the other 22 were aged 8–20 years. Continuation of root development was achieved in 6 cases, continuation and bridge formation occurred in 3 cases, bridge formation alone was seen in 21 cases and in 4 cases no root-end closure was observed. Hard tissue bridge formation was the most common form of root-end closure (Morfis & Siskos, 1991). In a similar study of 32 trauma-induced necrotic immature permanent incisors in 7–10-year-olds with or without a periradicular lesion, the mean duration for apical closure was 10 to 14 weeks (Lee et al. 2010). In a retrospective analysis of apical closure using CH in 15 non-vital immature incisor teeth, a success rate of 100% was reported within 1 year (Walia et al. 2000). Several variables were identified that influenced the time required for complete apexification. Older children with a narrow open apex had a shorter treatment time than younger children; teeth without periradicular infection showed some amount of root growth and apical closure that was faster than those with periradicular infection. Additionally, the investigators reported that the calcified bridge formed following apexification was a porous structure (Walia et al. 2000). Dominguez Reyes et al. (2005) examined the time taken to obtain apical closure in a sample comprised of 26 young permanent incisors with necrotic pulps and open apices. Apical closure was obtained in 100% of the cases studied. Of these, 88.4% needed three to four sessions of CH treatment (an average of 3.23 sessions) in order to obtain apical closure; the average time employed was 12.19 months. Preoperative symptoms and periradicular pathosis did not affect outcome (Dominguez Reyes et al. 2005). In a study of 28 necrotic incisors in children with an age range of 6 and 13 years, apical closure was achieved in all cases. The mean duration to achieve apical closure was 8.6 months with a range of 3.24 to 13.96 months. The type of tissue in the apical region was categorized as cementoid tissue (85.72%) or osseous tissue (14.28%). The teeth were monitored over a 2-year period following completion of the non-surgical endodontic treatment. In 7.1 % of the cases re-infection occurred (Mendoza et al. 2010).
Unfortunately, the Frank technique is sometimes unpredictable (Fig. 5.6). In addition to the various types of apical barriers formed, other inconsistencies relating to the use of CH for apexification include: the time for root apices to close, the number of dressings necessary to complete closure, and the role of infection. Depending on the study, the speed of barrier formation varies from 3 to 24 months (Frank 1966; Finucane & Kinirons 1999; Kinirons et al. 2001). There is also variation in the recommended number of reapplications of CH (Webber 1984; Yates 1988; Morse et al. 1990; Sheehy & Roberts 1997; Abbott 1998; Mackie 1998; Mackie & Hill 1999). There is no consensus on the impact of changing the dressing material on the rate and quality of apical barrier formation. Recommendations regarding the proper timing to change the CH have varied from monthly, once every 3 months, once every 6–8 months, to no change at all (Chosack et al. 1997; Sheehy & Roberts 1997; Abbott 1998; Kinirons et al. 2001; Felippe et al. 2005). Finally, there appears to be no consensus on the role of infection. Some studies report an increase in the time for apexification when infection is present (Cvek 1972; Kleier & Barr 1991) and others have demonstrated no statistically significant differences (Ghose et al. 1987; Yates 1988; Mackie 1998; Finucane & Kinirons 1999). Additionally, Torneck and Smith (1970) have indicated that there may be incomplete bridging of the apex even though a two-dimensional radiograph may give the appearance of complete bridging. Periradicular inflammation may persist around the apices of many teeth due to the presence of necrotic tissue in the crevices of the apical bridge. Therefore, the presence of a radiographically and clinically closed apex is not necessarily indicative of a normal periodontium (Koenigs et al. 1975).
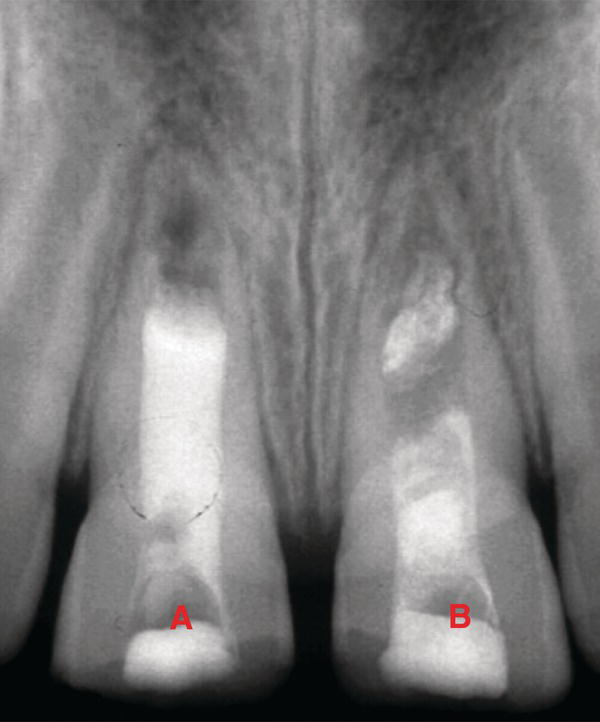
Fig. 5.6 Apexification with CH tooth: (A) has incomplete apical barrier formation; (B) has complete apical barrier formation.
Another major drawback of the apexification protocol using CH is the effect that a long-term application of CH has on the structural integrity of the root dentin. As previously stated, several studies have demonstrated that with longer exposures of dentin to CH, the dentin’s ability to with stand fracture is significantly decreased (Andreasen et al. 2002, 2006; White et al. 2002; Doyon et al. 2005; Rosenberg et al. 2007; Hatibovic-Kofman et al. 2008; Tuna et al. 2011; Bakland & Andreasen 2012).
NON-VITAL PULP THERAPY
Root-end closure via the use of apical barriers
Any treatment requiring several visits over a long period of time risks patient attrition caused by diminished tolerance to treatment and geographic relocation. If a child moves away during the course of treatment, it is difficult to ensure the dressing changes are made as needed until a barrier is formed. Likewise, patient compliance can be a problem when multiple visits are involved. Repeated visits to the dentist can be disruptive and difficult in a busy schedule so that the parent and child become wary of the routine and discontinue required visits (Heling et al. 1999). Also, appointments are easily forgotten because the patient usually has no discomfort and the tooth appears normal clinically. Another problem, which is avoided by one-visit apexification, is subjecting an unwilling child through treatments that may be very unpleasant to a young patient. Many children who fear trips to the dentist are even more traumatized by repeated visits. This response is even further heightened in younger children who present with wide open apices that require more treatment appointments. Given the complexity of apexification using CH, clinicians have frequently sought an alternative treatment protocol. Thus, the need for a reliable one-visit apexification treatment is clear.
Several investigators have demonstrated the use of dentin apical plugs in non-surgical root canal therapy of mature teeth (Tronstad 1978; Holland et al. 1980, 1983; Holland 1984; Brady et al. 1985). In 1967, Michanowicz & Michanowicz described a technique of using CH for an apical plug before gutta-percha filling in non-vital teeth with an open apex. Teeth with apical plugs of CH have also demonstrated mean leakage values that are significantly less than those obturated without plugs (Weisenseel et al. 1987). Pitts and associates (1984) histologically compared CH and dentin plugs in 36 overinstrumented canine teeth of nine mature cats. Their findings demonstrated that both plugs were effective in controlling the filling materials within the confines of the canals; however, a significant number of CH plugs washed out by 1 month. In contrast, the dentin plugs remained fully intact. Calcified tissue matrix formation was evident in the majority of the specimens containing dentin plugs after 1 month of placing them, whereas no foraminal calcification was seen until 3 months in samples treated with CH plugs. Tricalcium phosphate has also been proposed as an apical barrier in a single-appointment technique (Coviello & Brilliant 1979; Harbert 1991, 1996). When compared to multi-appointment CH–CMCP paste treatment of open apices, tricalcium phosphate resulted in no overfills in the apices of permanent human teeth. In contrast, CH treatment resulted in several cases that were associated with overextension of the root canal filling material. Because of its larger particle size, the experimental material offered improved resistance against compaction, and therefore, less material was extruded compared to the CH plugs (Coviello & Brilliant 1979). Brandell and associates (1986) evaluated the apical closures produced by demineralized dentin, hydroxyapatite, and dentin chips in monkeys. At the 6-month observation period, none of the demineralized dentin plugs showed complete apical closure. In contrast, 66% of the roots filled with hydroxyapatite exhibited complete apical closure, and 50% of roots with dentin chip plugs had complete apical closure. The authors concluded that the organic component of dentin might not be effective in inducing apical hard tissue formation.
Mineral trioxide aggregate apical plug
Mineral trioxide aggregate (MTA), whose principal components include tricalcium silicate, tricalcium aluminate, tricalcium oxide, and silicate oxide, has been commercially available for approximately 20 years. MTA was introduced to dentistry in 1993, primarily as a root-end filling material. While the majority of the studies involving MTA have noted its obvious benefits with its use in root-end fillings, these same attributes make MTA an attractive material for use in the treatment of immature non-vital teeth. The physical, chemical and structural aspects of this material are discussed in depth elsewhere within this text. MTA is a powder consisting of fine hydrophilic particles, which set in the presence of moisture, and result in a colloidal gel that solidifies to a hard structure (Torabinejad et al. 1995b). Numerous leakage studies (Torabinejad et al. 1993) have demonstrated that MTA leaks significantly less than many restorative materials, including amalgam, Intermediate Re/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


