Enamel
Composition, Formation, and Structure
Physical Characteristics of Enamel
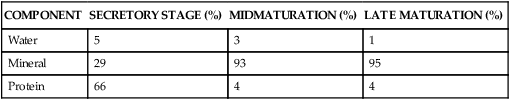
Fully formed enamel consists of approximately 96% mineral and 4% organic material and water (Table 7-1). The inorganic content of enamel is a crystalline calcium phosphate (hydroxyapatite) substituted with carbonate ions, which also is found in bone, calcified cartilage, dentin, and cementum. Various ions—strontium, magnesium, lead, and fluoride—if present during enamel formation, may be incorporated into the crystals. The susceptibility of these crystals to dissolution by acid provides the chemical basis for dental caries.
TABLE 7-1
Percentage Wet Weight Composition of Rat Incisor Enamel
| COMPONENT | SECRETORY STAGE (%) | MIDMATURATION (%) | LATE MATURATION (%) |
| Water | 5 | 3 | 1 |
| Mineral | 29 | 93 | 95 |
| Protein | 66 | 4 | 4 |
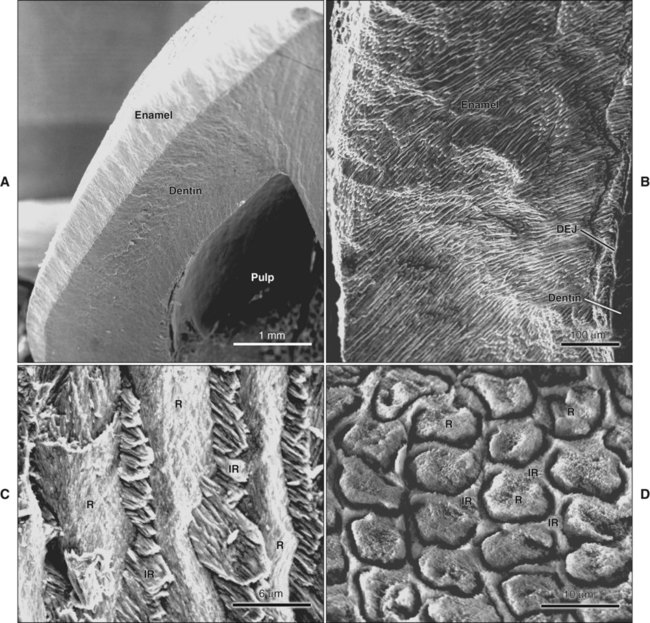
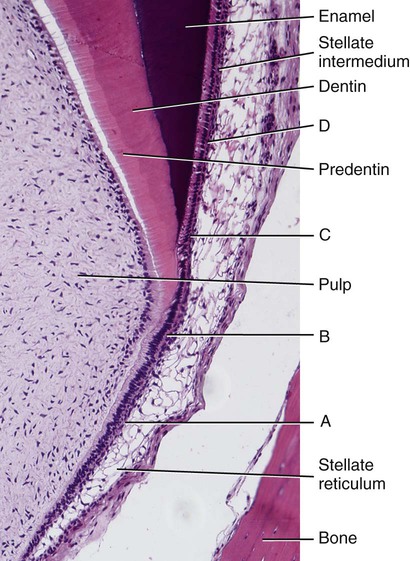
The high mineral content renders enamel extremely hard; this is a property that together with its complex structural organization enables enamel to withstand the mechanical forces applied during tooth functioning. This hardness also makes enamel brittle; therefore an underlying layer of more resilient dentin is necessary to maintain its integrity (Figure 7-1, A). If this supportive layer of dentin is destroyed by caries or improper cavity preparation, the unsupported enamel fractures easily.
Structure of Enamel
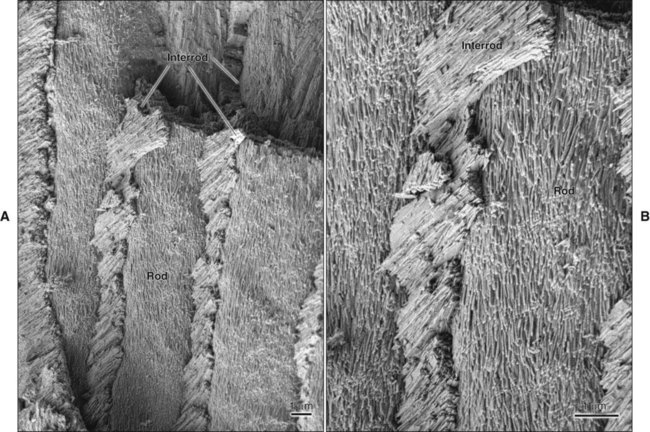
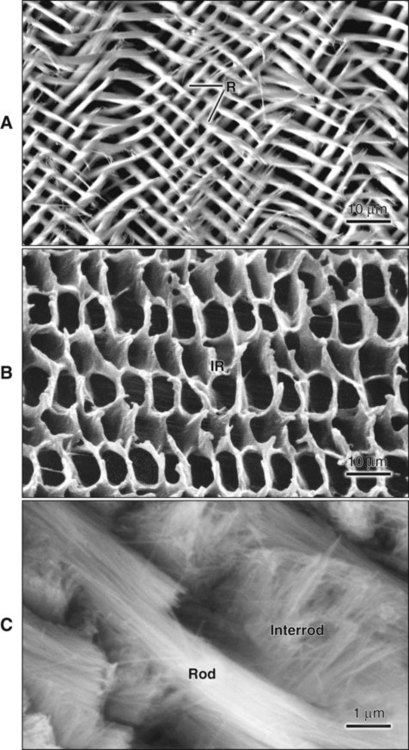
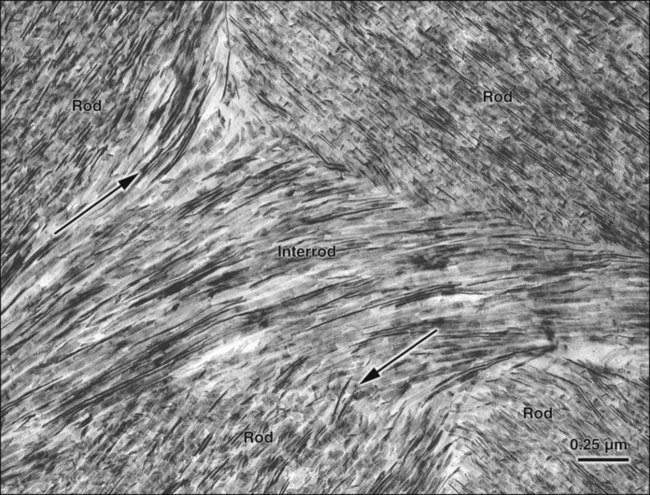
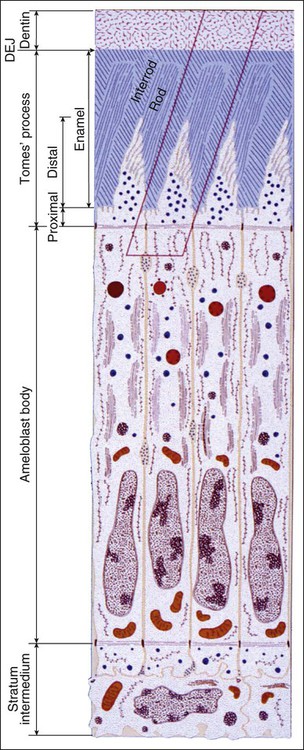
The fundamental organizational units of mammalian enamel are the rods (prisms) and interrod enamel (interprismatic substance) (Figure 7-2; see also Figure 7-1, B to D). The enamel rod was first described as hexagonal and prismlike in cross section, and the term enamel prism still is used frequently. This term is not used in this text because rods do not have a regular geometry and hence are not prismatic.
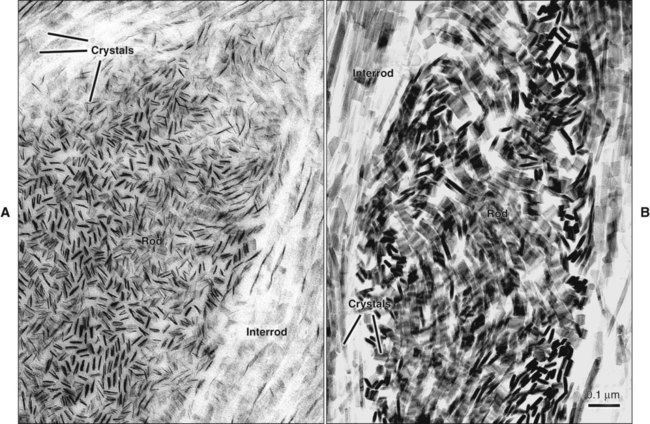
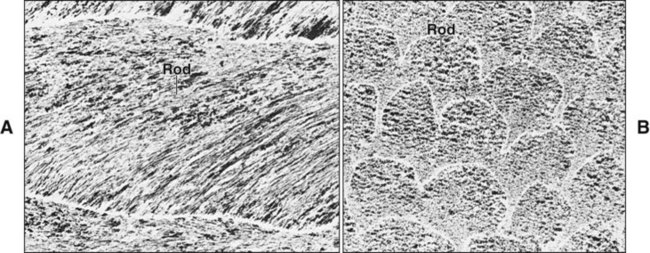
Enamel is built from closely packed and long, ribbonlike carbonatoapatite crystals (Figure 7-3; see also Figure 7-2) measuring 60 to 70 nm in width and 25 to 30 nm in thickness. The crystals are extremely long; some investigators believe that the length of the crystals actually spans the entire thickness of the enamel layer. The calcium phosphate unit cell has a hexagonal symmetry and stacks up to impart a hexagonal outline to the crystal, which is clearly visible in cross-sectional profile in maturing enamel (Figure 7-4). However, fully mature enamel crystals are no longer perfectly hexagonal but rather exhibit an irregular outline because they press against each other during the final part of their growth (Figure 7-5). These crystals are grouped together as rod or interrod enamel (see Figures 7-2 and 7-3).
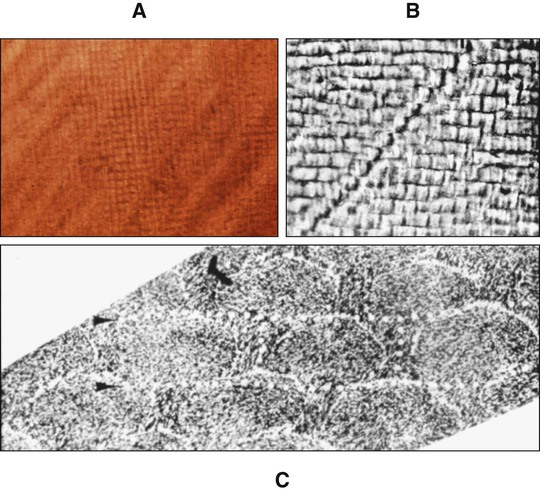
In ground sections the orientation of rods may be misinterpreted because the crystalline nature of enamel leads to optical interference as the light passes through the section, and their outline is difficult to resolve. As a result, when the roughly cylindrical enamel rods are sectioned, cut profiles that line up may be misinterpreted as rods viewed longitudinally, making an assessment of rod direction under the light microscope difficult (Figure 7-6). Use of both the scanning and transmission electron microscopes has overcome these interpretative difficulties.
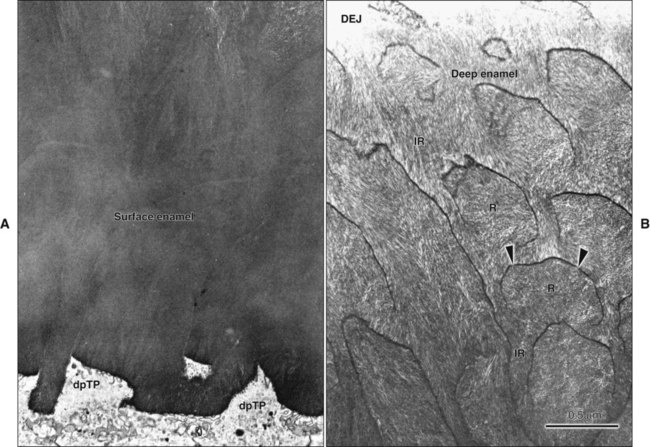
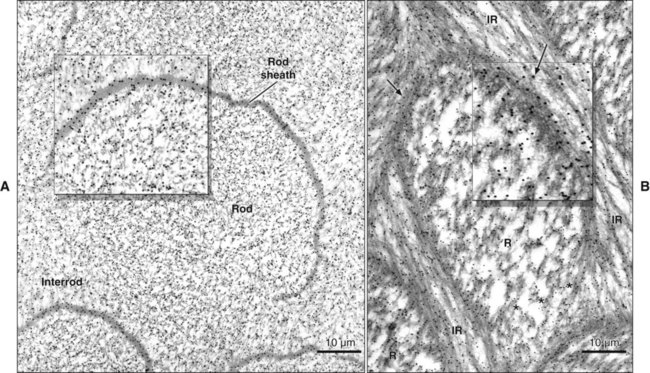
The rod is shaped somewhat like a cylinder and is made up of crystals with long axes that run, for the most part, in the general direction of the longitudinal axis of the rod (Figure 7-7; see also Figures 7-1, C and D; 7-2; and 7-3). The interrod region surrounds each rod, and its crystals are oriented in a direction different from those making up the rod (Figure 7-8; see also Figures 7-1, C and D; 7-2; 7-3; and 7-7). The difference in orientation is significant around approximately three fourths of the circumference of a rod. The boundary between rod and interrod enamel in this region is delimited by a narrow space containing organic material known as the rod sheath (Figure 7-9; see also Figures 7-1, D; and 7-8); the rod sheath is visualized more clearly in maturing enamels in higher mammals (Figure 7-10). Along a small portion of the circumference of the rod the crystals are confluent with those of interrod enamel (Figure 7-11; see also Figure 7-1, D). In this region, rod and interrod enamel are not separated and there is no space or rod sheath between them. In sections cut along the longitudinal axis of enamel rods and passing through the narrow region where rod and interrod are confluent, rod crystals can be seen to flare out into the interrod enamel (see Figure 7-8). The cross-sectional outline of these two related components has been compared with the shape of a keyhole. Because the keyhole analogy does not adequately account for some of the variations in the structural arrangement of the enamel components and is not consistent with the pattern of formation of enamel, this terminology has largely been discontinued. The basic organizational pattern of mammalian enamel thus is described more appropriately as cylindrical rods embedded in the interrod enamel.
Amelogenesis
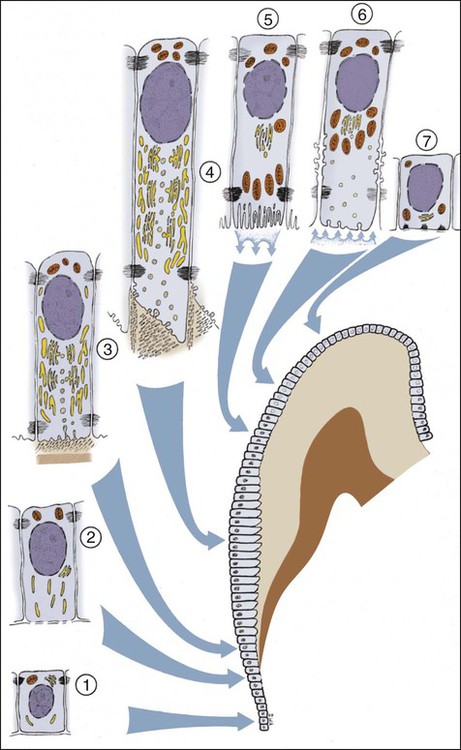
Amelogenesis, or enamel formation, is a two-step process. When enamel first forms, it mineralizes only partially to approximately 30% (Table 7-1). Subsequently, as the organic matrix breaks down and is removed, crystals grow wider and thicker. This process whereby organic matrix and water are lost and mineral is added accentuates after the full thickness of the enamel layer has been formed to attain greater than 96% mineral content.
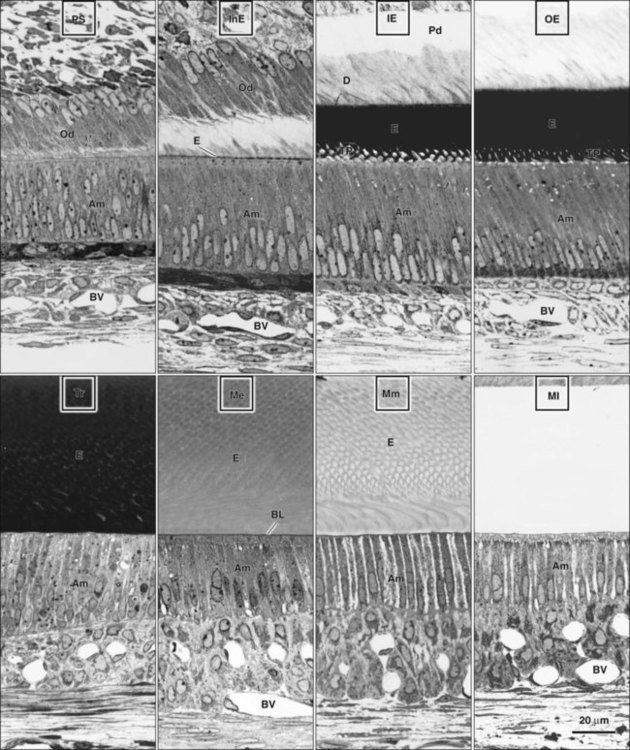
Ameloblasts secrete matrix proteins and are responsible for creating and maintaining an extracellular environment favorable to mineral deposition. This epithelial cell exhibits a unique life cycle characterized by progressive phenotype changes that reflect its primary activity at various times of enamel formation. Amelogenesis has been described in as many as six phases but generally is subdivided into three main functional stages referred to as the presecretory, secretory, and maturation stages (Figures 7-12 to 7-14). Classically, ameloblasts from each stage have been portrayed as fulfilling more or less exclusive functions. First, during the presecretory stage, differentiating ameloblasts acquire their phenotype, change polarity, develop an extensive protein synthetic apparatus, and prepare to secrete the organic matrix of enamel. Second, during the secretory stage (also called the formative stage), ameloblasts elaborate and organize the entire enamel thickness, resulting in the formation of a highly ordered tissue. Last, during the maturation stage, ameloblasts modulate and transport specific ions required for the concurrent accretion of mineral. Ameloblasts now are considered to be cells that carry out multiple activities throughout their life cycle and that up-regulate or down-regulate, some or all of them, according to the developmental requirements.
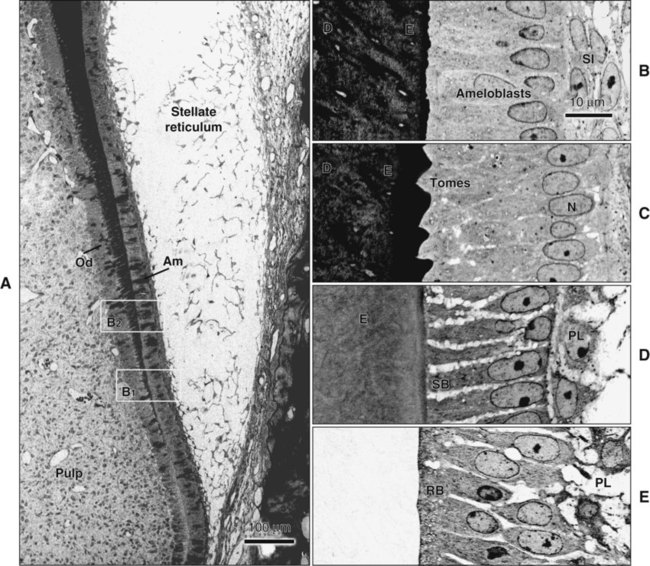
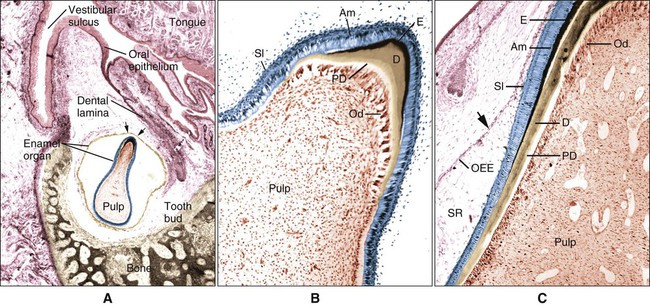
Enamel formation begins at the early crown stage of tooth development and involves the differentiation of the cells of the inner enamel epithelium first at the tips of the cusp outlines formed in that epithelium. The process then sweeps down the slopes of the tooth crown until all cells of the epithelium have differentiated into enamel-forming cells, or ameloblasts. Another feature is notable: when differentiation of the ameloblasts occurs and dentin starts forming, these cells are distanced from the blood vessels that lie outside the inner enamel epithelium within the dental papilla. Compensation for this distant vascular supply is achieved by blood vessels invaginating the outer enamel epithelium and by the loss of the intervening stellate reticulum, which brings ameloblasts closer to the blood vessels (Figure 7-15).
Light Microscopy of Amelogenesis
At the late bell stage, most of the light microscopic features of amelogenesis can be seen in a single section (Figure 7-16). Thus in the region of the cervical loop the low columnar cells of the inner enamel epithelium are clearly identifiable. Peripheral to the inner enamel epithelium lie the stratum intermedium, stellate reticulum, and outer enamel epithelium, the last closely associated with the many blood vessels in the dental follicle.
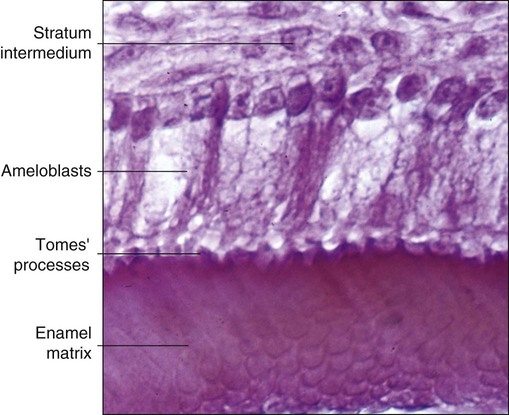
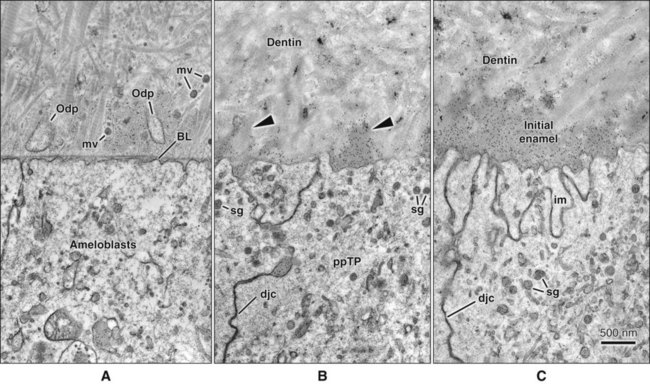
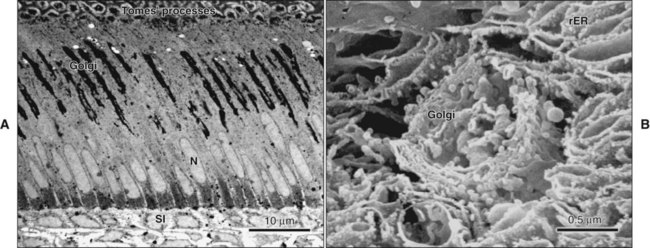
As the inner enamel epithelium is traced coronally in a crown stage tooth germ, its cells become taller and columnar, and the nuclei become aligned at the proximal ends of the cells adjacent to the stratum intermedium. Shortly after dentin formation initiates, a number of distinct and almost simultaneous morphologic changes associated with the onset of amelogenesis occur in the enamel organ. The cells of the inner enamel epithelium, now ameloblasts, begin to secrete more actively enamel proteins that accumulate and immediately participate in the formation of a partially mineralized initial layer of enamel (see Figure 7-12), which does not contain any rods. As the first increment of enamel is formed, ameloblasts move away from the dentin surface. Enamel is identified readily as a deep-staining layer in demineralized hematoxylin-eosin–stained sections (Figure 7-17; see also Figure 7-16). An important event for the production and organization of the enamel is the development of a cytoplasmic extension on ameloblasts, Tomes’ process (its formation and structure are described later in the chapter), that juts into and interdigitates with the newly forming enamel (see Figures 7-12 and 7-13). In sections of forming human teeth, Tomes’ processes give the junction between the enamel and the ameloblast a picket-fence or saw-toothed appearance (see Figure 7-17).
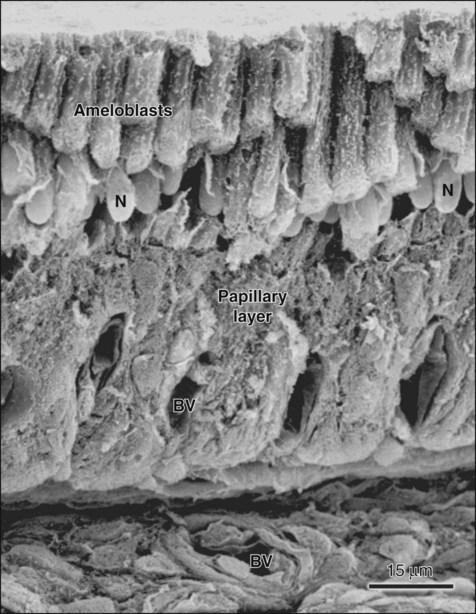
When formation of the full thickness of enamel is complete, ameloblasts enter the maturation stage (see Figures 7-12 and 7-13). Typically this stage starts with a brief transitional phase during which significant morphologic changes occur. These postsecretory transition ameloblasts shorten and restructure themselves into squatter maturation cells (see Figure 7-12). Cells from the underlying stratum intermedium, stellate reticulum, and outer enamel epithelium reorganize so that recognizing individual cell layers is no longer possible. Blood vessels invaginate deeply into these cells, without disrupting the basal lamina associated with the outer aspect of the enamel organ to form a convoluted structure referred to as the papillary layer (Figure 7-18; see also Figure 7-12).
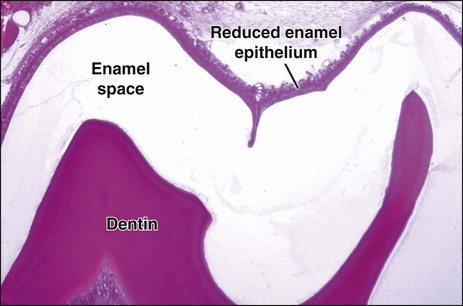
Finally, when enamel is fully mature, the ameloblast layer and the adjacent papillary layer regress and together constitute the reduced enamel epithelium (Figure 7-19). The ameloblasts stop modulating (discussed subsequently), reduce their size, and assume a low cuboidal to flattened appearance. This epithelium, although no longer involved in the secretion and maturation of enamel, continues to cover it and has a protective function. In the case of premature breaks in the epithelium, it has been proposed that connective tissue cells come in contact with the enamel and deposit a cementum-like material on it. During this protective phase, however, the composition of enamel can still be modified. For instance, fluoride, if available, still can be incorporated into the enamel of an unerupted tooth, and evidence indicates that the fluoride content is greatest in those teeth that have the longest interregnum between the completion of enamel formation and tooth eruption (at which time, of course, the ameloblasts are lost). The reduced enamel epithelium remains until the tooth erupts. As the tooth passes through the oral epithelium, the part of the reduced enamel epithelium situated incisally is destroyed, whereas that found more cervically interacts with the oral epithelium to form the junctional epithelium.
Electron Microscopy of Amelogenesis
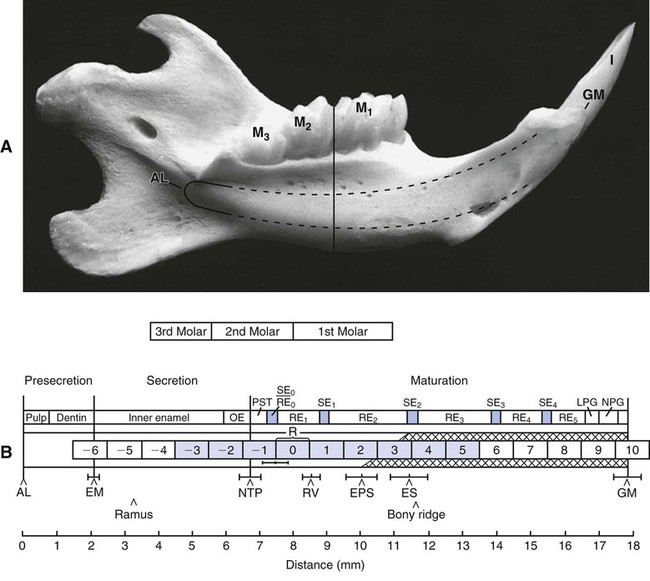
Ultrastructural studies of enamel formation by electron microscopy have added greatly to the understanding of this complex process. Such studies often have used the continuously erupting rat incisor as a model because all developmental stages can be found in a single tooth and because it has been demonstrated that the various stages of enamel formation bear an overall similarity to those in human teeth. In continuously erupting rodent incisors, the various stages of amelogenesis are disposed sequentially along the length of the tooth (Figure 7-20). In such a system, position represents developmental time, and one can look predictably at the various stages of amelogenesis by sampling at different positions from the apical end, where cell renewal occurs, to the incisal tip, where occlusal attrition balances the continuous, apically initiated tooth-forming activity.
Presecretory Stage
Morphogenetic Phase
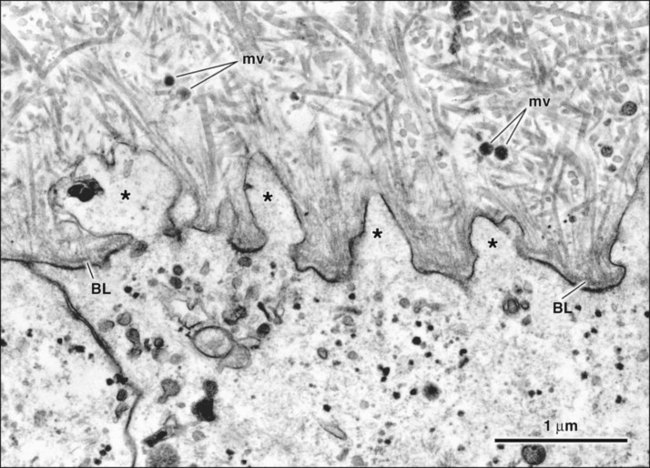
During the bell stage of tooth development, the shape of the crown is determined. A basal lamina is present between cells of the inner enamel epithelium and dental papilla (Figures 7-21 and 7-22, A). At this stage, the dentin is not yet mineralized as evidenced by the presence of intact matrix vesicles in it (Figure 7-23; see also Figures 7-21 and 7-22, A). The cells of the inner enamel epithelium still can undergo mitotic division, throughout the bell initially and eventually limited to the cervical portion of the tooth. These cells are cuboidal or low columnar, with large, centrally located nuclei and poorly developed Golgi elements in the proximal portion of the cells (facing the stratum intermedium), where a junctional complex exists. Mitochondria and other cytoplasmic components are scattered throughout the cell.

Differentiation Phase
As the cells of the inner enamel epithelium differentiate into ameloblasts, they elongate and their nuclei shift proximally toward the stratum intermedium. The basal lamina supporting them is fragmented by cytoplasmic projections and disintegrates during mantle predentin formation (see Figure 7-21). In each cell the Golgi complex increases in volume and migrates distally from its proximal position to occupy a major portion of the supranuclear cytoplasm. The amount of rough endoplasmic reticulum increases significantly and, in some species, mitochondria cluster in the infranuclear compartment, with only a few scattered through the rest of the cell. A second junctional complex develops at the distal extremity of the cell (facing differentiating odontoblasts), compartmentalizing the ameloblast into a body and a distal extension called Tomes’ process, against which enamel forms (Figure 7-22, B and C). Thus the ameloblast becomes a polarized cell, with the majority of its organelles situated in the cell body distal to the nucleus. These cells can no longer divide.
Although in the past these differentiating ameloblasts have been regarded as nonsecreting cells, it has been now clearly demonstrated that production of some enamel proteins starts much earlier than anticipated, even before the basal lamina separating preameloblasts and preodontoblasts is lost (see Figure 7-22, A). Surprisingly, preameloblasts also express dentin sialoprotein, an odontoblast product, albeit transiently. This reciprocal expression of opposing matrix proteins as cells differentiate, as well as the production of typical (ecto)mesenchymal proteins by enamel organ–derived cells at later stages (see Chapter 9), is consistent with the common ectodermal origin (oral epithelium/neural crest) of all hard tissue–forming cells in the craniofacial region.
From this point on up to the end of the secretory stage (see later), ameloblasts are aligned closely with each other, and attachment specializations, or junctional complexes, between them maintain the alignment. These complexes encircle the cells at their distal (adjacent to enamel) and proximal (adjacent to the stratum intermedium) extremities. Fine actin-containing filaments radiate from the junctional complexes into the cytoplasm of the ameloblasts, and can be distinguished as forming distal and proximal terminal webs (Figure 7-24). These junctional complexes play an important role in amelogenesis by tightly holding together ameloblasts and determining at different times what may, and what may not, pass between them to enter or leave the enamel.
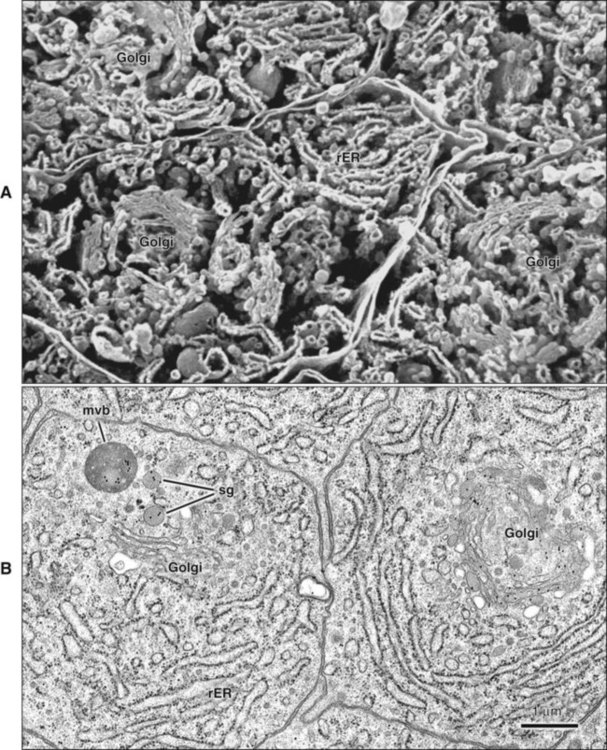
Secretory Stage
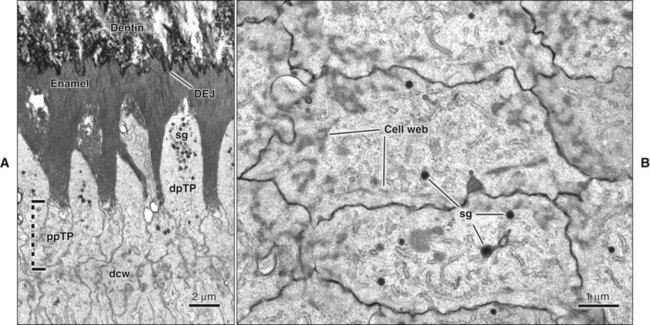
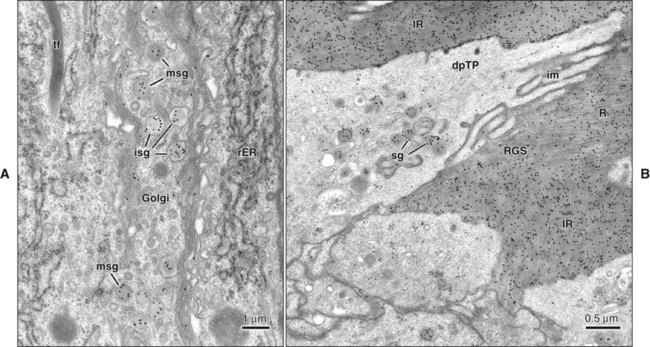
The fine structure of secretory stage ameloblasts reflects their intense synthetic and secretory activity. The Golgi complex is extensive and forms a cylindrical organelle surrounded by numerous cisternae of rough endoplasmic reticulum, occupying a large part of the supranuclear compartment (Figures 7-25 to 7-28, A). The messenger RNA for enamel proteins is translated by ribosomes on the membrane of the rough endoplasmic reticulum, and the synthesized proteins then are translocated into its cisternae. The proteins then progress through the Golgi complex for continued posttranslational modification (mainly for nonamelogenins) and are packaged into membrane-bound secretory granules. These granules migrate to the distal extremity of the cell, that is, into Tomes’ process (Figure 7-29, B; see also Figures 7-24, A; 7-27; and 7-28, B). Secretion by ameloblasts is constitutive; that is, it is continuous and secretory granules are not stored for prolonged periods, as is the case for salivary gland acinar cells, for example.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses