Nonpharmacologic Issues in Pain Perception and Control
Definition of Pain
Research is showing that the emotional and cognitive elements in the pain process have critical roles in the degree of pain awareness and, possibly more importantly, in the individual’s ability to induce self-control while enduring the suffering or its consequences.1 For instance, cognitive mechanisms (e.g., cognitive dissociation) that reduce the perceived quality and quantity of discomfort can be effectively taught to a patient before the experience of clinical or laboratory-induced pain. Children, through the process of suggestion, can be taught (to varying degrees) to ignore or to inhibit responsiveness to painful stimuli during cancer therapies.2,3
Theories of Pain Perception: Why and How It Is Experienced
Specificity
In the late 19th century, it was believed that the pain experience was merely a function of activating a particular set of neurons that resulted, metaphorically speaking, in a “ringing of the bell” at higher levels of the CNS signifying discomfort.4,5 The neural receptors (i.e., free nerve endings) and their pathways were considered to be specialized for the process of pain, just as other elements and pathways were rigidly designed as passages for other sensory information (i.e., pressure sensation).
Pattern
It was theorized that the recognition of painful stimuli by the individual was based primarily on the pattern of nervous activity that entered the CNS. Again, this theory was inadequate to explain the complexity of the pain experience, but it contributed significantly to the advancement of knowledge about pain mechanisms.4,5
Gate Control Theory
The gate control theory of pain (Figure 6-1) was developed by Melzack and Wall in 1965 and has been the most influential, comprehensive, and adaptive conceptualization of pain and its consequences to date. The essence of the theory proposes that various “gates” controlling the level of noxious input via small-fiber neurons to the spinal cord can be modulated by other sensory, large-fiber neurons; higher CNS input; or both. Postulated mechanisms for the gates include presynaptic inhibitory effects on secondary transmission cells in the spinal cord. In essence, this implies that large fibers (e.g., those for touch) can cause partial depolarization of the nerve terminals of small fibers (e.g., for pain) that innervate transmission cells in the spinal cord. This results in the release of fewer “packets” of neurotransmitter molecules and a decreased likelihood that transmission cells will summate and fire.
Transcutaneous electrical nerve stimulation (TENS), or the use of low-intensity electrical stimulation at peripheral sites, has been shown to relieve pain.6 Although the mechanism for TENS-mediated pain relief is not known, it has been suggested that its segmental effects may be due to activation of large-diameter, primary afferent fibers that in turn inhibit small-fiber transmission as predicted by the gate theory. A similar mechanism may account for the effects of acupuncture. TENS has been shown to produce partial analgesia to electrical tooth pulp stimulation in school-aged children.7
Proportionately more emphasis has been placed on the role and influence of higher CNS modulation of pain perception and reactivity.1,8,9 This reorientation is partially the result of more sophisticated studies suggesting the need for a more comprehensive explanation of cognitive, behavioral, and emotional influences in pain perception and control.10 Discoveries of endogenous opioids, widespread locations of opioid receptors throughout the CNS, and unnatural (i.e., electrical) stimulation of CNS sites resulting in pain threshold elevations also have sparked renewed interest in pain research.
In addition, animal studies have documented the presence and influence of descending supraspinal mechanisms on modulating painful input at the level of the spinal cord.11 Interestingly, a phenomenon called stimulation-produced analgesia (SPA) has been demonstrated in both humans and animals. SPA results in specific inhibition of pain or avoidance behaviors during electrical stimulation of discrete brain sites (e.g., periaqueductal gray area of the medulla) and has few side effects. SPA of the periaqueductal gray area has been shown to inhibit jaw-opening reflexes due to tooth pulp stimulation in cats.12
Neuromatrix
In more recent years the CNS, and more specifically those components of the CNS we generally refer to as higher levels of CNS functioning (i.e., above the midbrain), has become a popular focus of pain research. In one theory, a highly complex neuronal network or matrix purportedly exists and is referred to as the neuromatrix (Figure 6-2). The neuromatrix is thought to have some genetic basis but can be influenced by the internal and external environment.13 The uniqueness of the genetic makeup of the neuromatrix and its ultimate functional identity may aid in distinguishing each individual’s “body-self.” It is through this neuromatrix that cyclical processing and synthesis of neuronal activity may result in a characteristic pattern known as a neurosignature. It has been theorized that neurosignatures can help explain complicated states like pain in individuals who experience a specified set of symptoms that have environmental, situational, or other influential mediators (e.g., emotions). This abstract model undoubtedly will stimulate a focus of future research to aid in diagnosing, understanding, and explaining many clinical conditions.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 Outline
Outline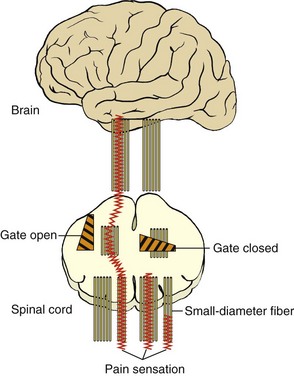
 FIGURE 6-1
FIGURE 6-1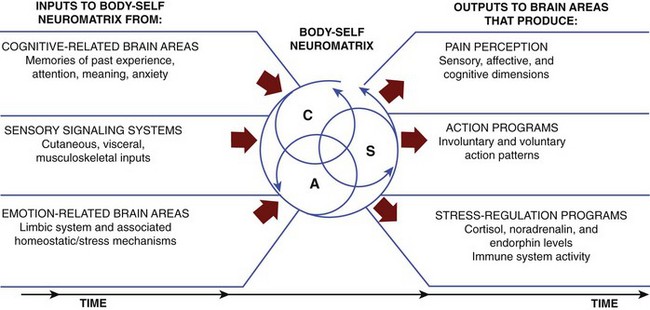
 FIGURE 6-2
FIGURE 6-2