CHAPTER 49 Antibiotic Prophylaxis
The discussion in this chapter is as evidence-based as possible in a field where evidence established by observational studies and randomized controlled trials is limited or nonexistent and where “expert” opinion has reigned often with total disregard for whatever evidence was available. Antibiotic prophylaxis has often been and is still used to “prevent” accusations that all was not done for the patient, in the hope of thwarting malpractice litigation. This practice has led to a gross overuse of antibiotics for defensive medicine with ensuing adverse effects and increased microbial resistance for which plaintiff’s attorneys have consistently refused to take responsibility. Often the justification for antibiotic prophylaxis to prevent metastatic or surgical infection is based on surrogate markers that do not reflect the true clinical situation. Antibiotic prophylaxis may reduce bacteremias associated with dental treatment, but that is not proof that this also reduces infective endocarditis (IE).93
The 2007 American Heart Association (AHA) Guidelines for the Prevention of Infective Endocarditis have assiduously reviewed all the evidence, particularly the alleged association of dental treatment procedures and IE and the efficacy of antibiotic prophylaxis for prevention of IE along with suitable recommendations based on current evidence.93 Some of that evidence is not repeated in this chapter, and the reader is encouraged to consult the original document.93 Other excellent and exhaustive studies have explored the basis for antibiotic prophylaxis for nonvalvular cardiovascular devices,9 prosthetic joints, and other situations associated with controversial antibiotic prophylaxis46,56 and, finally, the basis for it all—“the focal infection theory.”59
Concerns have been repeatedly expressed about the risk versus benefit for β-lactams as prophylactic agents and the risk of anaphylactic shock,58 particularly if antibiotic prophylactic prevention of IE does not work. In addition, data have now appeared showing that surgical prophylaxis in hospitals is associated with increased risk of Clostridium difficile infection,14 and hospital and community use of antibiotics enhances colonization by methicillin-resistant Staphylococcus aureus.85
PRINCIPLES OF ANTIBIOTIC PROPHYLAXIS
Antibiotic prophylaxis may be indicated if the infection to be prevented is common but not fatal or if it is rare but carries an unacceptably high mortality rate.6 The principles of antibiotic prophylaxis were established 30 to 40 years ago but have not often been appreciated.52,60,83,90 These principles are as follows: (1) satisfactory risk and cost/benefit ratios should exist in which the benefit to the patient significantly outweighs medical and financial risks, (2) the antibiotic must be in high concentrations at the target site (blood or tissue) before the onset of the bacteremia or surgery, (3) an antibiotic loading dose (two to four times the maintenance dose) must be used, (4) the antibiotic chosen should be effective against the most likely microorganism to cause the infection, and (5) the antibiotic is continued only as long as microbial contamination of or from the operative site continues.58,83,90
The adverse effects of antibiotic prophylaxis include (1) antibiotic allergy and toxicity, (2) superinfections (onset of a new infection while treating another infection), (3) selection of antibiotic-resistant organisms, and (4) induction of resistance gene transfer.56–58 Contraindications to antibiotic prophylaxis include the following: (1) an at-risk group cannot be sufficiently defined to prevent overuse and abuse of antibiotic prophylaxis, (2) efficacy of prophylaxis is too limited or unreliable, (3) the bacteremia to be prevented is too seldom a cause of infection, and (4) prophylaxis is directed at any and all potential microbial pathogens, rather than the colonization of a single pathogen.56,58 Antibiotic prophylaxis is primarily intended for two clinical situations: (1) to prevent metastatic bacteremias and (2) to prevent postsurgical infections. The science to support either of these situations is limited or essentially nonexistent.
PREVENTION OF METASTATIC INFECTIONS
With the advent of the 2007 AHA endocarditis prevention guidelines,93 the guidelines for the prevention of endocarditis of the Working Party of the British Society for Antimicrobial Chemotherapy (BSAC)24 and the guidelines of the National Institute of Health and Clinical Excellence (NICE) of Britain,82 the indications for IE prophylaxis have declined to a very few situations or, in the case of the NICE recommendations, to none. Box 49-1 lists these indications according to the AHA. There are none for NICE because this organization could find no evidence for any prophylaxis, including IE. Box 49-2 lists the general conclusions of the AHA, and Box 49-3 lists the cardiovascular and noncardiovascular conditions for which there is no evidence for benefit from antibiotic prophylaxis.
BOX 49-1 Recommendations of 2007 American Heart Association Endocarditis Prevention Guideline
From Wilson W, Taubert KA, Gewitz M, et al: Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia and the Quality Care and Outcomes Research Interdisciplinary Working Group, Circulation 116:1736-1754, 2007.
Dental Procedures for Which Endocarditis Prophylaxis Is Recommended
All dental procedures that involve manipulation of gingival tissue or the periapical region of the teeth or perforation of the oral mucosa*
Cardiac Conditions Associated with the Highest Risk of Adverse Outcomes from Endocarditis for Which Prophylaxis with Dental Procedures Is Reasonable
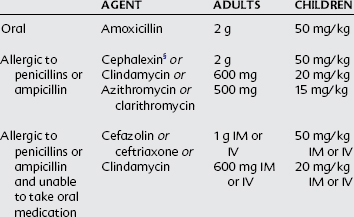
Oral Prophylaxis Regimens Before a Dental Procedure in Situations with High Risk
Single dose 30-60 minutes before procedure
CHD, Congenital heart disease; IE, infective endocarditis; IM, intramuscular; IV, intravenous.
* The following procedures and events do not need prophylaxis: routine anesthetic injections through uninfected tissue, taking dental radiographs, placement of removable prosthodontic or orthodontic appliances, adjustment of orthodontic appliances, placement of orthodontic brackets, shedding of deciduous teeth, and bleeding from trauma to lips or oral mucosa.
† Except for the conditions listed, antibiotic prophylaxis is no longer recommended for any other form of CHD.
‡ Prophylaxis is reasonable because endothelialization of prosthetic material occurs within 6 months after the procedure.
BOX 49-2 Conclusions of the American Heart Association
From Wilson W, Taubert KA, Gewitz M, et al: Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia and the Quality Care and Outcomes Research Interdisciplinary Working Group, Circulation 116:1736-1754, 2007.
Primary Reasons for Revision of Infective Endocarditis Prophylaxis Guidelines
Summary of Major Changes in Updated Document
CHD, Congenital heart disease; GI, gastrointestinal; GU, genitourinary; IE, infective endocarditis.
BOX 49-3 Medical Conditions for Which No Antibiotic Prophylaxis Is Recommended Before Dental Treatment
A systematic review of the literature by Lockhart and colleagues46 concluded that the evidence for antibiotic prophylaxis is essentially only “expert” opinion (author’s quotation marks) or case studies with general agreement that such prophylaxis is not useful or effective and in some cases potentially harmful. These clinical situations include native heart valves; prosthetic heart valves and pacemakers; hip, knee, and shoulder prosthetic joints; renal dialysis shunts; vascular grafts; immunosuppression secondary to cancer or cancer chemotherapy; systemic lupus erythematosus; and insulin-dependent type 1 diabetes.
A review by Baddour and coworkers9 concluded that there is no evidence to support antibiotic prophylaxis before dental treatment for patients with arterial grafts, cardiac pacemakers and implanted defibrillators, Dacron carotid patches, left ventricular assist devices, and peripheral or coronary artery stents. There is still considerable confusion regarding antibiotic prophylaxis before dental treatment in patients with various orthopedic prosthetic devices. The 1997 and 2003 Advisory Statements of the American Dental Association (ADA) and the American Academy of Orthopaedic Surgeons (AAOS) clearly advise that: “Presently no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prosthesis.”1,2 The confusion regarding the use of prophylaxis for certain immunocompromised patients and patients less than 2 years after device placement is discussed subsequently.
History of Endocarditis and Antibiotic Prophylaxis
Currently, most studies on antibiotic prophylaxis given before dental treatment have been shown to reduce, but not eliminate, these bacteremias. It is still unknown how many bacteria (inoculum size) are necessary to induce IE. It was also assumed that if the bacteremias were reduced, endocarditis would be prevented. There have never been any data to support this assumption; however, thousands of lawsuits have been filed alleging negligence against dentists with millions of dollars transferred from them and their insurance carriers to the dental patient allegedly developing IE. This hypothesis was expanded to promote antibiotic prophylaxis to prevent orthopedic joint prosthesis infections. Many other alleged metastatic diseases have supported the 100-year-old observation that: “Previously sclerosed endocarditis was in most cases due to mouth organisms.”8
This theory that metastatic bacteremias were the cause of anatomically distant diseases was embodied in the focal infection theory. At the turn of the 20th century, the focal infection theory proposed that a focus of infection (a confined area that contained bacteria) disseminated these microorganisms and their products in blood to distant body sites where a new infection arose.59 These foci of infection were primarily located in the mouth, tonsils, and gallbladder and were allegedly responsible for myriad diseases, including arthritis, neuralgias, myalgias, asthma, cancer, pancreatitis, thyroid disease, and “nervous diseases of all kinds.”59 After the loss of millions of teeth, tonsils, and gallbladders, the focal infection theory faded away in the 1930s and 1940s, only to be resurrected today with alleged causation of sarcoidosis, multiple sclerosis, amyotrophic lateral sclerosis, myasthenia gravis, Tourette’s syndrome, and other diseases by focal infections. The “oral-systemic connection” is another resurrection of the focal infection theory with as much “evidence” as in the past but with an ever-expanding group of associated diseases, including cardiovascular disease, preterm birth, diabetes mellitus, and Alzheimer’s disease.59
IE is a bacterial or fungal infection of one or more of the cardiac valves (aortic, mitral, tricuspid, or pulmonary) or the mural endocardium. The primary pathology is the formation of valvular vegetations composed of fibrin and platelets resulting from abnormal jets of blood that damage the valves over time and lead to the vegetations, which become infected by microorganisms in the blood (bacteremias, fungemias). Most IE is caused by staphylococci and streptococci owing to their ability to attach to surfaces using various adhesion molecules.93
The rationale for antibiotic prophylaxis to prevent IE with cardiac valvulopathy has been that (1) certain cardiac defects predispose to endocarditis, (2) most microbes causing IE are susceptible to antibiotics, (3) the risk of bacteremias is increased by certain invasive medical and dental procedures, (4) antibiotic prophylaxis reduces the incidence and magnitude of such bacteremias, and (5) antibiotics prevent bacterial attachment to damaged cardiac valves or their multiplication after they become attached.57,58,93 These suppositions remain valid for the greater risk of IE in individuals with damaged cardiac valves, but it is becoming increasingly obvious that routine bacteremic sensitivity to antibiotics is now unlikely, that it does not follow that reducing bacteremias reduces IE, and that bacteremias associated with daily living are much more likely to produce IE than a few dental appointments in a given year. The lack of evidence for dental treatment being the cause of IE, prosthetic joint infections (PJIs), brain abscesses (BAs), and other potentially bacteremic infections has been challenged since the mid-1970s by numerous individuals,55 and this has resulted in the new guidelines by the AHA, BSAC, and NICE. Reason and science have finally prevailed after 100 years.
EVIDENCE BASE FOR ANTIBIOTIC PROPHYLAXIS
Data Included in the American Heart Association Guidelines
The 2007 AHA statement on the prevention of IE has been an extensively reviewed document. Twenty-three members of the Writing Group; several content and foreign reviewers; and all members of the AHA Science and Advisory Group encompassing some 70 experts, including cardiologists, infectious disease specialists, and dentists, were involved. The AHA statement has been endorsed by the American College of Cardiology, the Infectious Diseases Society of America, the American Academy of Dermatology, the American Academy of Pediatrics, the International Society of Chemotherapy for Infection and Cancer, the Pediatric Infectious Disease Society, and the American Dental Association.93 The following discussion summarizes the findings of the AHA and presents relevant data.
Essentially, the guidelines state that there is no evidence that antibiotic prophylaxis is effective in preventing IE associated with dental treatment procedures, that the risk of bacteremias from daily living activities is magnitudes greater than that associated with dental treatment, and that such dental treatment procedures are a very small risk for IE. In addition, the guidelines clearly state the following: (1) there is no evidence that the incidence of IE is greater with a higher versus a lower magnitude of bacteremia, (2) the role of the duration of bacteremias is uncertain, (3) a presumed relationship between poor oral hygiene and IE risk is controversial, (4) bleeding from a dental procedure is an unreliable predictor of bacteremia, and (5) no data indicate that a reduction in bacteremia with amoxicillin reduces the risk of or prevents IE. The absolute risk rate for IE from a single dental procedure was between 100,000 and 1 million depending on the type and severity of the cardiac valvular pathology (Box 49-4).56,59,93
BOX 49-4 Absolute Risk Rate for Various Metastatic Focal Infections From a Single Dental Treatment Procedure
The absolute risk rate for endocarditis from a dental treatment procedure is essential to the determination of a risk/benefit assessment of antibiotic prophylaxis to prevent IE.56,59 One would need to premedicate 100,000 to 1 million patients to achieve one successful IE prevention, assuming that antibiotic prophylaxis works and dental treatment bacteremias cause 1% of VGS-induced IE (see Box 49-4).56 Because the mortality rate from VGS-associated IE is less than 10%, and the rate of anaphylactic death from penicillin is at least 1 per 1 million, it is likely that the risk of death is greater from the prevention than the disease.57,87
In 1975, Podgrell and Welsby64 published an estimate of the odds of IE occurring after a single dental procedure as 1 per 140,000. These data remained unappreciated until the popularization of evidence-based medicine. More specifically, the concept of absolute risk rates for disease incidence and prevalence determination were used to determine how many in a given population actually get the disease or benefit from its treatment. Steckelberg and Wilson78 published the absolute risk rates for IE in individuals with varying severity of cardiac valvular pathology. Using data from the United States about the annual dental visits per year (1.6), the annual incidence of community IE (11,200 cases), and the percent caused by VGS (25%), it was calculated that (1) the risk for VGS-caused IE in the general population, if all were caused by dental treatment–induced bacteremias, is 1 per 142,000 (very close to the Podgrell-Welsby estimate), and (2) if only 1% were caused by dental treatment, the odds were approximately 1 per 14 million.56,59
The risk rate increased substantially depending on the severity of the cardiac pathology using the Steckelberg and Wilson criteria.78 The odds for patients with (1) previous endocarditis are 1 per 95,000; (2) heart valve prostheses, 1 per 114,000; (3) rheumatic heart disease, 1 per 142,000; (4) congenital heart disease, 1 per 475,000; and (5) mitral valve prolapse with regurgitation, 1.1 per 1 million.56,59,78 It has likewise been calculated that the risk for PJIs from dental treatment was 1 per 2.5 million, and for BAs the odds ranged from 1 per 1 million to 10 million dental procedures.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses