CHAPTER 46 Antiseptics and Disinfectants
The historical importance for routine infection control procedures was underscored by epidemiologic investigations and other scientific evidence in the 1970s and 1980s. It was estimated at that time that an office treating 20 patients a day would encounter one active carrier of hepatitis B virus (HBV) every 7 days.9 This early finding, coupled with the fact that most microbial infections, including those caused by HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV), can be infectious before distinct signs and symptoms appear, makes the likelihood of unknowingly treating an infectious patient a certainty. Failure to treat every patient as potentially infectious—that is, with standard precautions, previously termed universal precautions—places the health care worker and all patients at needlessly increased risk of infection.5,7
The overall goals of infection control programs are as follows: (1) to reduce the numbers of pathogenic microorganisms to levels where patients’ normal defense mechanisms can prevent infection, (2) to break the cycle of infection and eliminate cross-contamination, (3) to treat every patient and instrument as capable of transmitting infectious disease, and (4) to protect patients and health care workers from infection and its consequences.7,15 The proper use of barrier techniques (gloves, mask, gown, eye protection, rubber dam), proper sterilization, disinfection, and antisepsis protocols accomplishes these goals.
It is important at the beginning of this chapter to understand the differences between the terms sterilization, disinfection, and antisepsis.8,28 Sterilization is the ultimate goal of any infection control protocol because it is the killing of all forms of microorganisms. To eradicate resistant viruses and bacterial endospores effectively requires the application of high heat or chemicals or both for a sufficient time. The most widely used means of attaining this objective in a dental office are dry heat, steam, and chemical vapor sterilization units. In medicine and industry, sterilization includes ethylene oxide and formaldehyde gases, ultraviolet and gamma radiation, and filtration. Disinfection is the application of chemicals to destroy most pathogenic organisms on inanimate surfaces. Although some chemicals used for disinfection are capable of achieving sterilization given sufficient time of exposure, their use to effect sterilization is discouraged because of the number of conditions that can lead to failure in this application. Antisepsis is the use of chemicals to destroy or inhibit pathogenic organisms on skin or living tissue. The difference between disinfection and antisepsis may seem small, but it leads to a wide divergence in the products used and the regulation of the products. Disinfectants fall under the regulatory authority of the U.S. Environmental Protection Agency and are subject to that agency’s rules for demonstration of effectiveness and use in the workplace. Antiseptics, because they are intended for application on living tissue, fall under the regulations of the U.S. Food and Drug Administration (FDA) regarding effectiveness and clinical use.
Numerous treatment area surfaces can become contaminated with saliva, blood, and other potentially infectious substances during provision of care. The routine use of chemical disinfectants and disposable supplies has become historically more appropriate in certain instances because it is neither possible nor necessary to sterilize all contaminated items or surfaces. This trend is especially applicable in dentistry, where many instruments and environmental surfaces become contaminated with saliva and blood during routine procedures.8,9 Organisms contained in these fluids include staphylococci, streptococci, Mycobacterium tuberculosis, cytomegalovirus, herpes simplex virus (HSV), HBV, HCV, HIV, and a number of upper respiratory tract viruses such as influenza and rhinoviruses. Environmental surfaces in particular do not lend themselves to sterilization and must be cleaned and disinfected or covered with disposable barriers.5,7,11
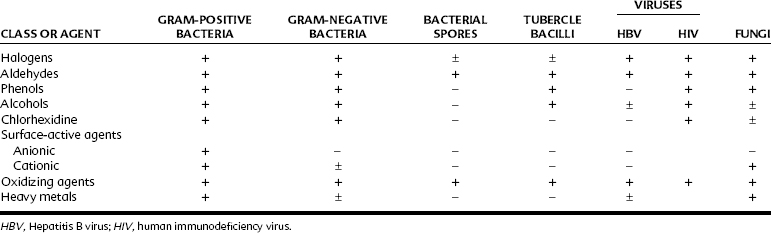
Table 46-1 lists representative classes of compounds used as disinfectants or antiseptics with their effectiveness against various representative organisms. The aldehyde and certain halogen-based and oxidizing compounds have the broadest range of effectiveness. These agents also tend to be the most toxic to human tissue. Consequently, their use has been primarily limited to disinfection. The other chemical classes are less effective antimicrobial agents, but also tend to be less harmful to human tissue and find use as disinfectants and antiseptics. Some distinguishing features of the chemical groups are listed in Table 46-2, and their major clinical uses are noted in Table 46-3.
| AGENT | ACTIVITY | LIABILITIES |
|---|---|---|
| Chlorine dioxide | Rapid disinfection activity; can be used for sterilization with 6 hr of exposure | Corrosive; activity greatly reduced in the presence of protein and organic debris; requires good ventilation |
| Glutaraldehyde | As 2%-3.2% immersion preparation, broad-spectrum antimicrobial activity; sporicidal after 10 hr of contact; long use life | Very irritating to skin and mucous membranes; allergenic with repeated exposures |
| Hypochlorite | Rapidly acting, broad-spectrum bactericidal, sporicidal, virucidal disinfectant | Irritating to skin; corrosive; can degrade some plastics |
| Iodophors | Rapidly acting, broad-spectrum bactericidal disinfectant; residual antimicrobial activity remains on surface after drying | Corrosive to some metals; may discolor some surfaces; inactivated by hard water |
| Phenols | Broad-spectrum antimicrobial activity; effective in presence of detergents | Can degrade plastics; irritating to skin and eyes; inactivated by hard water and organic debris |
TABLE 46-3 Miscellaneous Uses of Disinfectants and Antiseptics
| AGENT | FORMULATION (WEIGHT/VOLUME) | USE |
|---|---|---|
| Alcohol | 70% | Solvent and adjuvant for other agents; prevention of bedsores |
| Parachlorophenol | Variable | Root canal debridement |
| Phenol | 0.5%-1.4% | Relief of sore throat |
| Eugenol | Variable | Relief of pulpal pain |
| Guaiacol | Variable | Relief of pulpal pain |
| Sodium hypochlorite | 5% solution | Root canal debridement |
| Iodine solution | 8%-9% iodine | Plaque-disclosing solution |
| Povidone-iodine | Solution with 1% available iodine | Plaque-disclosing solution |
| Formaldehyde | 4% (10% formalin) | Fixative for tissue biopsy specimen |
| Hydrogen peroxide | 3% | Wound cleaning |
| 30% | Tooth bleaching |
HALOGENS AND HALOGEN-RELEASING COMPOUNDS
Halogens and halogen-releasing compounds include some of the most effective antimicrobial compounds used for disinfection and antisepsis. Their primary mode of action seems to depend on the free halogen reacting covalently with key microbial enzyme systems.10 Despite many years of research and use, the exact mechanism is unknown, although reactions with sulfhydryls and disulfides within proteins seem to be the most likely sites of action. Chlorine and iodine have historically been the most useful and effective halogens.
Chlorines
The salts (Na+, Ca++, and Li+) of hypochlorite, in the form of chloride lime, have been used since the mid-1800s as a source of chlorine for disinfection and as an antiseptic. Because of the irritating nature of sodium hypochlorite formulations, they are currently used primarily as disinfectants. This halogen primarily functions as an antimicrobial in the form of hypochlorous acid, into which it is rapidly converted in water. Elemental chlorine is a potent germicide and kills most bacteria in 15 to 30 seconds at concentrations of 0.10 to 0.25 ppm.10 The presence of a base in commercial preparations of sodium hypochlorite helps to stabilize the hypochlorite, which first must be converted to hypochlorous acid before it can release the chlorine. Useful dilutions for surface disinfection range from 1 : 10 to 1 : 100 in water, with exposure times of 10 to 30 minutes.5 Sodium hypochlorite surface disinfectants have an efficacious, broad antimicrobial spectrum dating back to the 1970s, when a 1 : 10 dilution of bleach in water was shown to be effective against HBV in hospitals. Disadvantages of bleach solutions include a strong tendency to corrode metals, an odor that some people find offensive, and the need for diluted disinfectant solutions to be prepared fresh daily. Current commercially available diluted hypochlorite disinfectants are more stable and remain active longer than earlier formulations. In addition, even though they are destroyed during disinfection, tubercle bacilli seem to be more resistant to hypochlorite compared with other common pathogens.25
Iodine and Iodophors
The development of iodophors—iodine or triiodide complexed with natural polymers such as polyvinyl pyrrolidone or polyether glycols—led to the application of iodine-containing preparations as antiseptics and surface disinfectants. One of the reasons for this application is their additional capability as surfactants, allowing them to be used as excellent cleaning agents. Iodophors have little or no odor, increase the solubility of iodine, are less allergenic than tinctures of iodine, reduce discoloration of surfaces, and provide a reservoir for sustained halogen release. Compared with aqueous solutions with the same total iodine concentration, the concentration of free molecular iodine (the active antimicrobial agent) is lower in iodophor preparations. This liability is offset by the release of iodine from the polymer complex as the free iodine, which reacts with microorganisms. When used with a spray-wipe-spray technique, iodophor disinfectants are efficient cleaning agents and effective surface disinfectants.8,28
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses