CHAPTER 45 Antiplaque and Antigingivitis Agents*
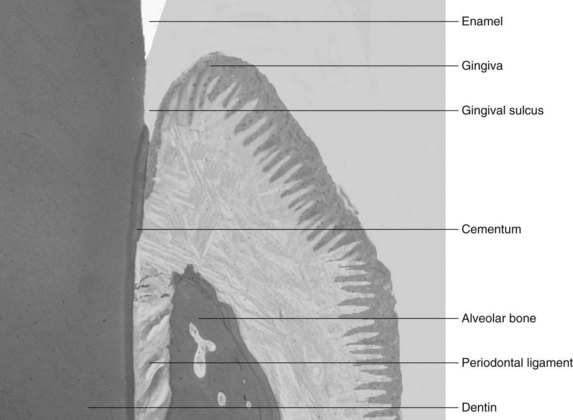
The periodontium, which is responsible for the retention of teeth in the maxilla and mandible, consists of four different tissue types. Cementum and alveolar bone are the hard tissues to which the fibrous periodontal ligament anchors the tooth into the skeleton, and the gingiva is the covering tissue of the periodontium (Figure 45-1). The gingiva is a unique body tissue because it allows the penetration of calcified tissue (i.e., teeth) into an intact mucosa, while protecting the underlying periodontal tissues. The buildup of infectious organisms on these structures give rise to some of the most common diseases in humans.
The accumulation of microorganisms on the tooth surface along the gingival margin can alter the structure and function of the gingiva, inducing an oral inflammatory reaction; clinically, this is known as gingivitis.64 During adolescence, the occurrence of gingivitis is almost universal, and in adulthood, it affects approximately 50% of the population.2 Because of the frequent appearance of gingivitis, this disease remains a principal concern for the dentist, as it can be converted to other, more destructive forms of periodontal disease.64 The prevention or cure of gingivitis is of particular interest to dentists.
Dental caries is another common oral disease the prevalence of which varies in regard to the tooth surface and age of the individual.25 Although caries is a worldwide problem associated with dental plaque and refined carbohydrates, some individuals, particularly individuals in lower educational, lower socioeconomic, and older age groups, are at greater risk.25
RATIONALE FOR BIOFILM DRUG THERAPY
Many different types of materials accumulate on teeth. The most ubiquitous and important deposit is dental plaque or dental biofilm. Dental biofilm consists primarily of microorganisms in an organized matrix of organic and inorganic components.85 Bacteria account for at least 70% of the mass of the biofilm; 1 mm3 of dental biofilm contains more than 100 million bacteria consisting of more than 400 different species.41,69 The organic matrix of biofilm consists of polysaccharide, protein, and lipid components, whereas the inorganic matrix is primarily composed of calcium and phosphorus ions.85
Gingivitis is due principally to the accumulation and retention of dental biofilm coronal to the gingival margin.45,62 The accumulation of supragingival biofilm is also a prime influence in the development of the subgingival biofilm.16 As undisturbed biofilm matures, it changes in composition and becomes more complex. A bacterial succession occurs whereby microorganisms associated with gingival health (i.e., gram-positive rods and cocci) are replaced by microorganisms associated with gingivitis (i.e., gram-negative cocci and rods) and spiral-shaped organisms and spirochetes. As a consequence of the change in microflora, inflammation-induced changes in the gingiva cause an increase in epithelial cell turnover and connective tissue degradation, resulting in anatomic changes that tend to deepen the gingival sulcus causing a gingival pocket to form.37 This change in gingival architecture and the subgingival environment provides a new and better protected niche for bacteria to grow. Here, they are continually bathed by exudate from the gingival crevice and end products from the supragingival biofilm. Control of supragingival biofilm also has a profound influence on the developing composition of periodontitis-associated subgingival biofilm.37
Dental caries is a chronic disease that is characterized by the progressive decalcification of tooth structure. The biofilm contains bacterial species (e.g., Streptococcus mutans) that convert refined carbohydrates to lactic acid and other acids.103 These acids can dissolve tooth mineral, resulting in a subsurface lesion initially and a cavity if the process continues over time. The unimpeded progress of dental caries can penetrate the enamel or cementum and progress through the dentin to the dental pulp. When the dental pulp is affected, a pulpitis develops resulting in tooth pain (i.e., toothache).
Some current commercially available therapeutic measures for control of biofilms include agents that act directly on the microflora and agents that interfere with bacterial attachment or the mechanical removal of the biofilm or both. Discussion of mechanical techniques is beyond the scope of this chapter, but excellent comprehensive reviews on mechanical plaque control can be found elsewhere in the dental literature.15,29
PHARMACOKINETICS OF THE ORAL CAVITY
Absorption
The vascularity of the oral cavity, combined with a thin epithelial lining in some areas, allows for the absorption of drugs at a rapid rate.43,94 Nonionized drugs, such as nitroglycerin, take advantage of these tissue characteristics and diffuse rapidly across the oral mucosa into the bloodstream. In contrast to most drugs, for which the principal objective is to introduce the agent into the bloodstream rapidly, the goal of oral topical agents is to be retained in the oral cavity for as long as possible.35 Rapid oral absorption can lead to toxic effects elsewhere in the body and a significant reduction of the free drug in the oral cavity. In most instances, the drugs used to restrain plaque levels are highly ionized and are generally unable to penetrate the oral mucosa.
Distribution
When an agent is topically applied in the oral cavity, the free drug can act at the primary site (i.e., bacteria in the plaque), or it can be partitioned to compartments where the drug binds nonspecifically. These drug reservoirs include the enamel, dentin, and cementum of the tooth; the oral mucosa; the organic and inorganic components of plaque; and salivary proteins.20
The fraction of the administered dose that is nonspecifically bound to oral reservoirs depends on the concentration, amount of time, and chemical nature of the agent used. A 1-minute rinse with 0.2% chlorhexidine results in approximately 30% of the total amount dispensed being retained after 1 hour, whereas a 3-minute rinse with 0.1% sodium fluoride results in less than 1% of the administered dose being found in the oral cavity after 1 hour.31 The ability of oral agents to bind nonspecifically and reversibly to oral reservoirs is an important quality for a sustained release of drugs to occur.
Clearance from the Oral Cavity
Salivary flow is crucial in the removal of many agents from the oral cavity. Human saliva has a diurnal flow that varies from 500 to 1500 mL of secretion in the daytime to less than 10 mL of secretion at night.5 The rate of clearance of a drug from the oral cavity is of profound importance in determining the duration of time a drug is in contact with the tooth surface.20
Substantivity
The time that a drug is in contact with a particular substrate in the oral cavity is defined as substantivity.104 Drugs that have a prolonged duration of contact are considered to have high substantivity.105 In the oral cavity, substantivity depends on two important pharmacokinetic features: (1) the degree of reversible, nonspecific binding to oral reservoirs and (2) the rate of clearance by salivary flow (Figure 45-2).
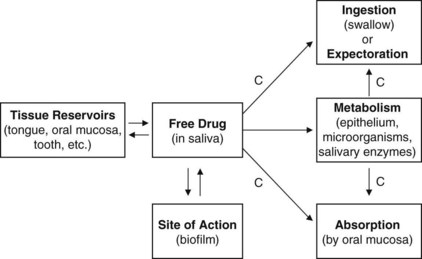
FIGURE 45-2 Pharmacokinetic factors that affect substantivity of agents. C, Clearance from the oral cavity.
Oral reservoirs are an important source for the continued release of drugs. The oral compartments that accumulate a drug must be able to bind reversibly large portions of the administered dose and release therapeutic concentrations of free drug to the site of action over long periods. Effective agents with high substantivity ideally would not bind irreversibly or with high affinity to oral reservoirs.20,31
Salivary flow also significantly affects the substantivity of topically applied liquid agents. The clearance of an agent from the oral cavity is directly proportional to the rate of salivary flow. During periods of high salivary flow, there would need to be a greater release of drug from oral reservoirs to maintain therapeutic concentrations.20,31 Strategies that use natural or drug-induced periods of low salivary flow can increase the substantivity of an oral agent.
IDEAL PROPERTIES OF ANTIPLAQUE AGENTS
The pursuit of an ideal agent to reduce dental biofilms has been an ongoing search in dentistry for centuries.107 In 1890, Willoughby D. Miller, an American dental surgeon, stated in his now-famous book, The Micro-Organisms of the Human Mouth, that “we ought to be able by means of properly chosen antiseptic material … to prevent as well as arrest microbial-induced diseases in the oral cavity.”67 Since that time, numerous antiplaque mouth rinses have been introduced to the public, many with dubious claims, and only more recently have dentists been able to prescribe therapeutically effective agents. Considering that the average time a person spends mechanically removing plaque from teeth is approximately 37 seconds,106 having a “chemical” toothbrush would be a great benefit to improve oral health.
The principal properties of an ideal antiplaque agent include efficacy, stability, low clearance, safety, and taste (Box 45-1). An antiplaque agent must be able to suppress meaningfully or eliminate specific pathogens with no untoward local or systemic side effects. It should not allow the overgrowth of opportunistic organisms or encourage the development of resistant organisms. When used, it should be slowly released over time in the oral cavity with continued antimicrobial effect. The agent should be stable at room temperature and have a color and taste that is pleasing to the consumer. Last but not least, it should be relatively inexpensive to purchase. No such perfect antiplaque agent exists today.
The myriad claims regarding antiplaque efficiency of drugs has led the American Dental Association (ADA) Council on Scientific Affairs19 to develop guidelines for testing the long-term efficacy of chemotherapeutic products for control of supragingival dental plaque and gingivitis. The requirements of these guidelines are summarized in Box 45-2 and have been adopted, in some cases with modifications, by the U.S. Food and Drug Administration (FDA), the Canadian Dental Association, and the British Dental Association. To be considered acceptable, a product should be tested in at least two, independently conducted, 6-month clinical trials in populations that represent individuals for whom the product is intended.
ANTIPLAQUE AND ANTIGINGIVITIS AGENTS
Bis-biguanides
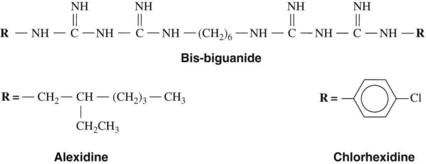
The bis-biguanides, chlorhexidine and alexidine, are cationic agents with fungicidal activity and bactericidal action against gram-positive and gram-negative organisms. Chlorhexidine is a chlorophenyl biguanide (Figure 45-3) that has been used as the acetate and, more commonly, the gluconate salt (which is more soluble) in mouth rinses, gels, and dentifrices for control of plaque and gingivitis. It binds to anionic groups on the bacterial surface, probably the phosphate groups of teichoic acid in gram-positive bacteria and the phosphate groups of lipopolysaccharides in gram-negative bacteria. When the bis-biguanide binds to the organism, the cell’s membrane becomes permeable, allowing the cytoplasmic contents to leak. At higher concentrations, chlorhexidine causes precipitation of cytoplasmic proteins. By virtue of their cationic properties, the bis-biguanides also bind electrostatically to hydroxyapatite of teeth, to acquired pellicle, to plaque, and to buccal mucosa.
In one of the earliest studies on the dental applications of chlorhexidine, Schroeder91 showed a 73% reduction of supragingival calculus plaques formed on carrier foils in short-term (3-day) tests. Subsequently, Löe and Rindom Schiøtt61 showed that chlorhexidine was the most effective antiplaque and antigingivitis agent that had been tested until that time. In short-term trials with an experimental gingivitis model, a twice-daily rinse with 0.2% chlorhexidine gluconate completely prevented accumulation of plaque and the onset of gingivitis. These observations have been confirmed in numerous trials in humans and animals. Chlorhexidine mouth rinse in this experimental model prevented the development of white-spot lesions associated with incipient caries.63
The efficacy of chlorhexidine mouth rinse as an antiplaque/antigingivitis agent is dose-dependent in the range of 0.03% to 0.2%.6,55 The volume and frequency of use and the concentration are important in determining the clinical response.57 Although no significant difference in response was found between a 0.2% and a 0.12% chlorhexidine mouth rinse when administered in a 15-mL dose twice daily (delivering a total of 60 mg and 36 mg of the agent),92 a significant difference in response was found between a 0.2% and a 0.1% chlorhexidine mouth rinse when administered in a 10-mL dose twice daily (providing 40 mg and 20 mg of the agent).6 Additional factors, such as bioavailability of the formulation, may also affect the dose response.
Long-term use (6 months) of chlorhexidine mouth rinses has shown plaque reduction and significant prevention of gingivitis in children57 and adults.38 In a short-term study (21 days), 0.12% chlorhexidine mouth rinse used twice daily was clearly effective in reducing plaque (62% to 99%) compared with placebo, whereas rinsing with a phenolic compound containing essential oils, or sanguinarine with zinc chloride, resulted in no significant reduction in plaque.95 The chlorhexidine rinse was superior to the other agents in its ability to maintain optimal gingival health during the entire 3 weeks the mouth rinses were used. Similarly, a 0.2% chlorhexidine rinse was approximately twice as effective as a sanguinarine rinse in a 19-day nonbrushing study in which plaque and gingivitis scores were assessed.70 Fluoride (100 ppm), when combined with chlorhexidine (0.12%), does not interfere with the antiplaque/antigingivitis activity of a mouthwash.49 The ADA Council on Scientific Affairs has accepted a mouth rinse containing 0.12% chlorhexidine gluconate as a safe and effective adjunct to brushing and flossing and regular professional care in helping prevent and reduce supragingival plaque and gingivitis.18
Extensive safety testing of the short-term and long-term effects of these compounds shows extremely low levels of toxicity locally and systemically. The low toxicity is a result of the poor absorption of chlorhexidine by the oral cavity and gastrointestinal tract, resulting in a limited amount entering the bloodstream. When chlorhexidine directly contacts mammalian connective tissue cells, there is usually a harmful effect. In cell culture, chlorhexidine can adversely affect gingival fibroblast attachment to root surfaces.14 Protein production in human gingival fibroblasts was reduced at chlorhexidine concentrations that would not normally affect cell proliferation.65 Such findings corroborate earlier studies showing delayed wound healing in standardized mucosal wounds after rinsing with a 0.5% chlorhexidine solution.8 No teratogenic or reproductive changes have been found.
Bis-biguanides are effective as antiplaque and antigingivitis agents. They should not be used prophylactically, but as therapeutic agents for patients with active disease. This use requires proper diagnosis and supervised care until the disease is controlled. In the United States, these agents are for prescription use only. Chlorhexidine mouth rinses can serve as an important adjunct to regular oral hygiene for short-term application, particularly in the healing phase after periodontal surgery, oral surgery, and insertion of immediate dentures and for the treatment of acute necrotizing ulcerative gingivitis.55 Chlorhexidine rinses can also be used for intermittent short-term application three to four times a year to prevent repeated denture stomatitis, limit plaque and gingivitis in patients with dental implants, and suppress the salivary titers of S. mutans in patients with high caries activity. Finally, long-term use of such a mouth rinse on a daily, weekly, or biweekly basis may benefit special patients with agranulocytosis, leukemia, hemophilia, thrombocytopenia, kidney disease, bone marrow transplantation, or acquired immunodeficiency syndrome (AIDS); patients being treated with cytotoxic or immunosuppressive drugs or radiation therapy; and patients who are physically handicapped or mentally retarded (Box 45-3).
Investigators have tested more intensive, professionally applied antimicrobial treatment with varnishes containing high concentrations of chlorhexidine compounds: 5%, 10%, 20%, and 40%.54,82-84,86-89 The goals were to suppress S. mutans for an extended period, to prevent the increase of S. mutans normally accompanying placement of fixed orthodontic appliances, and possibly to eliminate them from the mouth. In these studies, S. mutans was successfully suppressed and in some cases eliminated for up to 22 months. There was no long-term effect on Actinomyces species or Streptococcus sanguis, however. A 40% chlorhexidine varnish, applied to exposed root surfaces of patients who had had periodontal surgery, was as effective as a fluoride varnish in preventing root caries.87
Nonionic Bisphenols
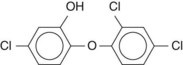
Triclosan is a broad-spectrum antimicrobial compound whose chemical name is 2,4,4′-trichloro-2′-hydroxydiphenyl ether (Figure 45-4).90 Originally, triclosan was used extensively in soaps, antiperspirants, and cosmetic toiletries as a germicide.90 Currently, triclosan has been incorporated into toothpaste and mouth rinse because of its wide spectrum of antimicrobial effects, anti-inflammatory actions, and only modest toxicity.66
Triclosan is active against a broad range of oral gram-positive and gram-negative bacteria.90 The antibacterial actions of triclosan were originally thought to affect cell membrane integrity by binding to membrane targets and interfering with transport mechanisms.90 More recent work has shown that the effects of triclosan in bacteria occur by inhibiting the enzyme enoyl-acyl carrier protein redu/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses