CHAPTER 44 Anticaries Agents*
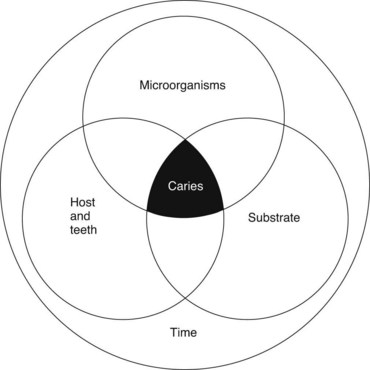
Dental caries is a pathologic process of microbial etiology that results in localized destruction of tooth tissues. From an anatomic and microbiologic perspective, there are several different types: pit and fissure caries, smooth surface caries, root caries, and deep dentinal caries. The process of tooth destruction involves dissolution of the mineral phase, consisting primarily of hydroxyapatite crystals, by organic acids produced by bacterial fermentation. To appreciate the activities of anticaries agents, it is imperative to understand that the initiation and progression of caries is based on the principle of remineralization versus demineralization as part of a dynamic continuum. The resulting balance between these two directly controls the effusion and diffusion of minerals into and out of the enamel lattice. Figure 44-1 outlines protective and pathologic factors for dental caries.25 The biologic basis of dental caries involves three principal factors: the host, particularly the saliva and teeth; the microflora; and their substrate, the diet. In addition, a fourth factor, time, must be considered in any discussion of the causes of caries. These factors can be portrayed as four overlapping circles (Figure 44-2).
Fluorine is a member of the halogen family. It is the most electronegative of all the elements, which makes it extremely reactive. Fluorine combines with almost every element. It is also reactive with organic radicals. It is rarely found in the free state in nature but is widely distributed as fluorides* in the earth’s crust, ranking seventeenth in abundance (0.06% to 0.09%). It usually occurs in minerals such as fluorspar (CaF2), cryolite (Na3AlF6), or fluorosilicates (Na2SiF6) and in rocks in the form of mica, hornblende, and pegmatite. In biologic mineralized tissues, such as bones and teeth, it occurs as an impure apatite crystal, not as fluorapatite (Ca10[PO4]6F2). The lattice of biologic apatite crystals contains many impurities, either in the lattice itself or adsorbed on the surface.81 Carbonate ions (2% to 5%) substitute for some phosphate ions; some Ca++ is substituted by other ions, such as Na+, K+, Mg++, and Zn++; and some hydroxyl ions are substituted by fluoride. The approximate representation of the formula of this apatite is Ca10−x(Na)x(PO4)6−y(CO3)z(OH)2−u(F)u. Although only some of the hydroxyls of the apatite lattice are substituted by fluoride (i.e., u is much smaller than 2), this change profoundly alters the resistance of enamel to demineralization.
Previous theories held that systemically acquired fluoride (pre-eruptive) was of prime importance in caries prevention and that it was unnecessary to continue the use of fluoridated water after the enamel had calcified.52 Subsequent findings clearly showed a benefit, however, of posteruptive or topical fluoride exposure; in children in some communities that stopped fluoridating the water or in children who moved away from fluoridated communities, caries rates increased. More recently, some investigators have argued that posteruptive or topical fluoride effects are of sole importance in caries prevention and that systemic benefits are minimal.24,47 Careful analyses of caries epidemiology in teeth according to their eruption time, as related to the onset of water fluoridation, have revealed, however, significant pre-eruptive and posteruptive beneficial effects.30,77 Approximately two thirds of the greatest reductions in pit and fissure caries came from pre-eruptive fluoride, whereas in smooth surfaces the decrease was only 25%. In approximal surfaces, half the reduction was from pre-eruptive fluoride, and half was from posteruptive fluoride.31 Maximal caries-preventive effects of fluoridated water were achieved by optimal pre-eruptive and posteruptive exposure of all surfaces types.77
SYSTEMIC FLUORIDE
Fluoridation of Communal Water Supplies
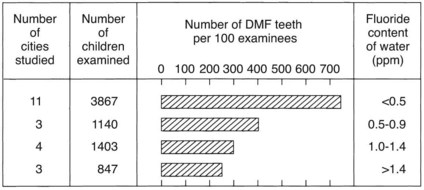
Classic epidemiologic surveys of the prevalence of dental caries, carried out by Dean and others during the late 1930s and early 1940s, showed an inverse relationship between caries prevalence and fluoride concentration in drinking water. Initially, these surveys were limited to school-age children residing in different cities with naturally high or low fluoride concentrations in the public water supplies (Figure 44-3). Subsequently, it was shown that adults and children who have continually consumed fluoridated water lose fewer teeth and have lower incidences of decayed, missing, and filled teeth. Of increasing importance regarding geriatric dentistry is the finding that lifelong residence in communities with naturally occurring fluorides is associated with a significant reduction in the prevalence of root caries or root fillings in the population.6,79
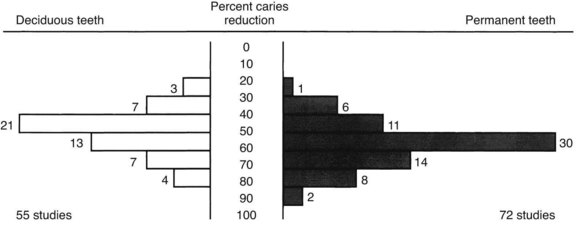
Dental fluorosis (discussed later) has been directly related to the concentration of fluoride in the drinking water. An optimal level of fluoride in the water supply provides significant protection against caries yet entails minimal risk of fluorosis. The optimal concentration depends on the annual average maximum daily air temperature in the community (temperature influences the amount of water ingested). In temperate climates, where the annual average maximal daily air temperature is 14.7° C to 17.7° C (58.4° F to 63.8° F), the optimal level of fluoride is 1 ppm. Carefully controlled independent studies conducted during the 1940s-1960s have shown that if fluoride is added to the domestic water supply to bring it up to optimal levels (controlled water fluoridation), decay could be reduced by 50% to 60% (Figure 44-4). These clinical trials were conducted in the United States and Canada, which were the first countries to initiate such programs, and in diverse populations in Australia, Hong Kong, Ireland, Germany, The Netherlands, New Zealand, and the United Kingdom. More recently, because of the widespread daily use of topical fluoride and the ingestion of fluoride-containing foods and beverages made in fluoridated communities, the difference in caries prevalence between fluoridated and nonfluoridated communities has been observed to be 15% to 40% depending on the age group and area examined.59
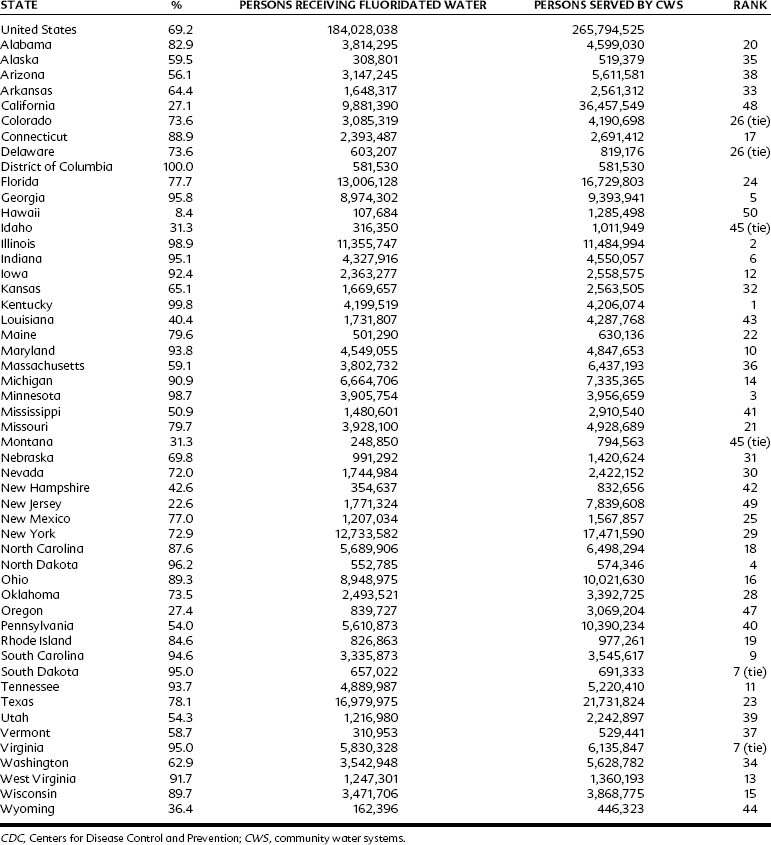
In some regions of the United States, a high proportion of the population is living in optimally fluoridated communities, so that the minority of the population where the water fluoride is suboptimal may be getting significant amounts of fluoride from food and beverage products processed in the optimally fluoridated areas, yielding a “diffusion” or “halo” effect on caries reduction. Failure to account for the diffusion effect may result in underestimation of the total benefit of water fluoridation, especially in high-diffusion exposure regions.29 Studies in Canada have documented the processing of beverages, especially soft drinks, in fluoridated communities and their distribution in nonfluoridated areas.14 The halo effect does not uniformly apply throughout the United States, however. Marked regional differences exist; in a 2006 Centers for Disease Control and Prevention (CDC) report on populations receiving optimally fluoridated public drinking water, it was noted that 54% of the population in Utah received optimally fluoridated public drinking water, a 148% increase from a similar 2002 report, whereas regionally close Nevada reported 72% of its population receiving optimally fluoridated public drinking water, an increase of 4%.10 The state-by-state fluctuations are presented in Table 44-1. In the United States, approximately 184 million individuals (69% of the population) were provided with optimally fluoridated drinking water.10 These totals were also higher than those reported in 2002 (162 million, 66%).3 Worldwide, more than 300 million individuals are now consuming water that either is adjusted to or naturally contains an optimal concentration of fluoride.
Opponents of water fluoridation have questioned its safety, yet careful comparisons of communities with optimal versus suboptimal concentrations of fluoride in water supplies have found no significant difference in the frequency of birth defects or in mortality statistics (including deaths from heart disease, cancer, and stroke). Optimal fluoridation of drinking water does not pose a detectable cancer risk to humans, as evidenced by extensive human epidemiologic data.86 Thorough medical examinations of children in fluoridated and nonfluoridated communities were undertaken in some of the initial studies of controlled water fluoridation; no significant differences in health or in growth and development were found. One study was quite detailed and included tonsillectomy rates; height and weight; onset of menstruation; bone density by x-ray examination of hands and knees; skeletal maturation; blood hemoglobin titer; erythrocyte and leukocyte count; urinalysis; and skin moisture, texture, color, and eruptions.74 The conclusion of this long-term pediatric study was that the reduction in caries was accompanied by no indication of any adverse effect from the use of fluoridated water.
Some concern has been raised about a possible relationship between fluoride in the water supply and frequency of hip fractures. Of several studies, two showed a protective relationship, four found no relationship, and three reported an increased relative risk. These conflicting findings are caused by the multifactorial pathogenesis of osteoporotic fractures (cigarette smoking, having a small thin frame, history of previous fracture, excessive alcohol intake, estrogen deficiency, physical inactivity) and may prove impossible to resolve by current epidemiologic-ecologic methods.43 The collective results of all these studies on hip fracture rates have yielded relatively small or no associations or have had weak statistical power and do not provide a basis for altering public health policy regarding water fluoridation.27 An expert committee of the World Health Organization concluded, “With respect to hip fracture and bone health, there is no scientific evidence for altering current public health policy on the use of fluorides for caries prevention.”23 Finally, a meta-analysis of articles on fluoridation and bone fracture published between 1966 and 1997 found that the relative risk was 1.02. It concluded that water fluoridation has little protective or deleterious effect on fracture risk.40
Another area that has garnered much speculation and public attention has been the theorized relationship between fluoride exposure and osteosarcoma. Bassin and colleagues7 suggested a potential positive correlation between fluoridated drinking water and osteosarcoma in males. This study had several notable weaknesses, which included being largely interview-based and subject to recall bias, associating fluoridated community drinking water with actual consumed fluoride, and lack of biologic analysis of fluoride concentrations in bone. Bassin and colleagues7 are quick to emphasize that further studies that directly evaluate fluoride uptake and address confounding variables are required before any preliminary conclusions can be made.4 More detailed discussion of some of the claimed health risks of water fluoridation can be found elsewhere.34,60
Opponents have focused more recently on the use of fluorosilicic acid and its Na+ salt, which together account for 91% of the fluoridating agents used by American water works.84 They have asserted that the fluorosilicate ion (SiF6−) promotes the solubilization of lead from the distribution system, increasing the lead concentration in the tap. In addition, they believe that residual fluorosilicate is responsible for reducing gastric pH and converting particulate lead to bioavailable lead ion, increasing its uptake in the bloodstream.50,51 Supposedly such higher blood lead concentrations account for aggressive and violent behavior. Although the kinetics of the dissociation and hydrolysis of fluorosilicate are poorly understood, all the rate data suggest that equilibrium should have been achieved by the time water reaches the consumer’s tap, if not by the time it leaves the water plant.84,85 There is no proof that the ingestion of lead or its bioavailability is increased.
Fluoride Supplements
Communal water fluoridation is the best method for providing systemic fluoride because the benefits accrue automatically without any conscious effort required. Where water fluoridation is not feasible because of individual wells (approximately 20% of the U.S. population), or where political opposition, apathy, or lack of funds prevent its implementation (approximately 30% of the U.S. population), supplements offer an alternative source of systemic fluoride. Fluoride tablets, drops, and lozenges have been proved unequivocally to be effective cariostatic agents, provided that such supplements are taken on a daily basis continuously from birth to approximately 16 years of age. The cariostatic effects of fluoride supplements have ranged from less than 10% to more than 80%, generally depending on how soon after birth supplementation starts and on the degree of compliance.19 The highest caries reductions have been reported in private pediatric practices in which there is a high degree of motivation on the part of the professionals who prescribe the supplements, the parents who give the supplements to their young children, and the children themselves as they get older and become responsible for taking the supplements. When distribution of fluoride supplements has been attempted on a large scale, such as by community health centers, well-baby clinics, and county health departments, long-term compliance has been poor. An estimated 16% of U.S. children younger than 2 years used fluoride supplements in 1986,61 but compliance tends to decrease in older children. There has been some debate on the efficacy of fluoride supplementation, and a 2008 systematic review commissioned by the American Dental Association (ADA) concluded that “during the first 3 years of life, however, there is only limited evidence regarding the effectiveness of fluoride supplements in preventing caries.”39
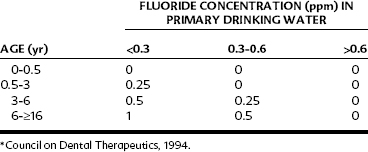
Professional fluoride supplementation should ideally be based on a caries-risk assessment.1 The correct dosage in prescribing fluoride supplements depends on two factors: the age of the child and the existing fluoride concentration in the water supply (Table 44-2). The latter information can be obtained from the local water supply authority (except in the case of private well water). Failure to determine the fluoride concentration in the communal water source can result in a fluoride overdosage and consequent dental fluorosis. For young infants, drops are more convenient than tablets because they can be directly dispensed into the child’s mouth with a medicine dropper or added to foods (e.g., cereals) or beverages (e.g., milk, formula, or juices). For older children whose primary teeth have erupted, fluoride tablets or lozenges are indicated, which provide systemic benefits when swallowed and topical benefits as they are chewed and swished around the teeth. Fluoride tablets or lozenges are available in 0.25-mg, 0.5-mg, and 1-mg strengths. No more than 120 mg of fluoride (264 mg of sodium fluoride) should be dispensed in any one container, which should be provided with a childproof top and labeled: “Caution—store out of reach of children.” A sample prescription of a fluoride supplement for a 2-year-old residing in a community with 0.1 ppm of fluoride in the water supply is shown in Figure 55-5.
Because fluoride supplements are taken as a single bolus that causes a rapid elevation in blood fluoride concentrations, most studies have identified them as a major risk factor for dental fluorosis.73 Current fluoride prescription practices have undergone close scrutiny, and it is generally agreed that a reduction in dosage is indicated in the age period from birth to 6 years because this is the period when the permanent anterior teeth are vulnerable to dental fluorosis (the “window of vulnerability”). A reduced dosage schedule of fluoride supplements has been accepted by the ADA, American Academy of Pediatric Dentistry, and American Academy of Pediatrics, as shown in Table 44-2.2 In the 6-month to 3-year age cohort, it is recommended that fluoride drops be prepared in a more dilute form, such as containing 0.25 mg of fluoride in 0.25 mL (instead of in a single drop), to minimize overdispensing errors at home. In Canada, a more drastic reduction in dosage—with no supplements until age 3 years; 0.25 mg at ages 3, 4, and 5 years; and 1 mg from age 6 years—has been recommended.13
Insufficient data exist to establish the efficacy of prenatal supplements given to pregnant mothers in reducing caries in offspring. The U.S. Food and Drug Administration (FDA) does not permit any fluoride preparation to be labeled, represented, or advertised for prenatal use. Only a small portion of the enamel of some of the primary teeth (mostly of the incisors) has entered the stage of secondary mineralization (maturation stage) at birth and almost no permanent teeth except the tips of the first molars (which are at the formative stage).17 It is more important to ensure that adequate fluoride supplements are taken regularly after birth. The concentration of total fluoride in human milk is approximately 0.05 ppm and in cow’s milk, approximately 0.1 ppm. Both types of milk are negligible sources of fluoride. Although an infant gets very little fluoride from a mother’s milk, in most cases there is no need to supplement breastfed children who reside in optimally fluoridated communities. Because the average duration of nursing in the United States is only 4 months, the amount of fluoride obtained from optimally fluoridated water supplies used in preparing formula and baby food suffices. If an infant resides in a suboptimally fluoridated community, the dosage schedule shown in Table 44-2 should be followed.
TOPICAL FLUORIDE
Not all fluoride agents and treatments are equal. Different fluoride compounds, different vehicles, and vastly different concentrations of fluoride, ranging nearly 100-fold, have been used with different frequencies and durations of application (Table 44-3). All these variables can influence the clinical outcome regarding caries prevention and management. The efficacy of topical fluoride in caries prevention depends on the concentration of fluoride used, the frequency with which it is applied and probably the duration of application, and, to some extent, the specific fluoride compound used.48,57,58
TABLE 44-3 Range of Therapeutic Fluoride Concentrations in Topical Agents Used to Prevent Caries
| METHOD/VEHICLE | FLUORIDE CONCENTRATION (ppm) |
|---|---|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses