Diagnosis and Management of Endodontic-Periodontic Lesions
Anatomic Considerations of the Pulpal and Periodontal Continuum
Persistent infection in the pulp tissue leads to secondary infection and breakdown of tissues in the periodontium. Conversely, severe periodontal disease may initiate or exacerbate inflammatory changes in the pulp tissue. This mutuality of infection between pulp and periodontium is mediated through physical routes, allowing for communication between the two structures. The main and obvious route of communication is the apical foramina. Advanced pulpitis will lead to pulp necrosis, which often is accompanied by inflammatory bone resorption at the root apex, as found in cases of apical periodontitis or an apical abscess (Figure 43-1). This is also known as retrograde periodontitis because it represents the periodontal tissue breakdown from an apical to a cervical direction and is the opposite of orthograde periodontitis that results from a sulcular infection. This is typically identified as a periapical radiolucency (PARL) (Figure 43-2). Retrograde periodontitis is the most common example of pulpal diseases leading to secondary periodontal breakdown. The presence of patent apical foramina can also lead to pulpal inflammatory changes secondary to a severe periodontitis in cases where the periodontal defect reaches the apical foramina.
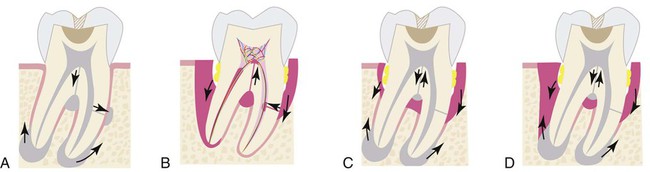
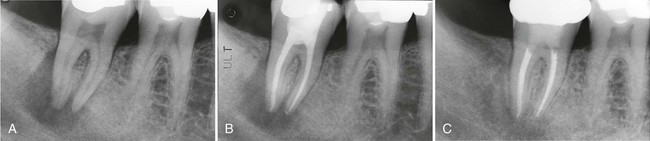
Alternatively, lateral or accessory canals may also be the route of periodontal and pulpal communications. Prevalence of accessory root canals in various human teeth and their contribution to the complexity of the root canal system have been well established. Accessory canals are found along the length of the root canals, albeit to varying frequencies depending on their location. Earlier studies, using the “clearing technique” for transparent root canal visualization, showed that 59.5% of maxillary second premolars possess lateral canals; 78.2% of those are located in the apical regions of the root canals.156 Notably, accessory canals were also found in both midroot and cervical regions, albeit with reduced frequencies at 16.2% and 4.0%, respectively. A subsequent study showed that 28.4% of permanent molars exhibit patent accessory canals in furcation regions,51 suggesting that these accessory canals allow a pulpal and periodontal communication to exist. Root canal therapies fail frequently in maxillary molars because of unidentified second mesial canals. These canals are found in a surprisingly high percentage (80.8%) of teeth.67 Clearly, accessory canals can lead to asymptomatic apical periodontitis resulting from chronic pulpal diseases. This can be readily detected in periapical radiographs (Figure 43-3) and the periodontal lesions usually heal after the successful completion of endodontic therapy. Questions also arise as to whether pulpal disease can develop from periodontal infections through accessory root canals. Kirkham76 reported that out of 100 permanent human teeth extracted as the result of severe periodontal disease, only 2 teeth possessed accessory canals within the periodontal pockets. Thus, the likelihood that primary periodontal infections will reach the dental pulp through accessory canals is rare.
The third route of communication between the periodontium and the pulp is through the dentinal tubules. Dentinal tubules maintain a tapered structure along the length from the pulpodentinal complex (PDC) to the dentinoenamel junction (DEJ) with the diameter of 2.5 µm at PDC and 0.9 µm at the DEJ.149 It is therefore conceivable that dentin is a permeable structure, and that the permeability changes at different locations along the root surface according to the size and density of the dentinal tubules. Bacterial colonization in the tubules from infected root canals has been well documented.132 Also, bacterial invasion into dentinal tubules from the periodontal pocket has been demonstrated,50 suggesting that dentinal tubules may allow pulpal irritation from chronic periodontal infections.
Dentin permeability through dentinal tubules is a clinically important issue. The permeability may be measured through hydraulic conductance described earlier.121 Subsequently, investigators have studied the effects of various agents and stresses on dentin permeability. Root planing, as part of routine periodontal therapy, for example, is shown to decrease dentin permeability and result in the formation of a smear layer that is acid-labile.38 However, dentin permeability may increase on removal of the smear layer, resulting in tubular penetration of oral pathogens and subsequent pulpal irritation. Further study is necessary to delineate the role of dentinal tubules in causing a secondary infection in either the pulpal or periodontal tissues. Clinicians need to be cognizant of the fact that patent dentinal tubules can provide effective irritant conduits between these two otherwise distinct tissues.
Factors Initiating Pulpal and Apical Diseases
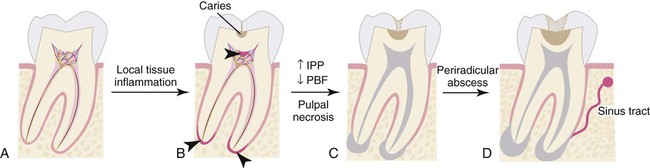
Pulpal and apical diseases are initiated by numerous external factors that may include microorganisms, trauma, excessive heat, restorative procedures, restorative agents, and malocclusion. These insults lead to inflammatory changes in the pulp, starting from a reversible or irreversible pulpitis and ultimately progressing to pulpal necrosis and subsequent breakdown of the periodontium. Dental caries is a prominent cause of pulpal disease, and bacterial infection is the primary form of microbial insult to the pulp. A systematic review of literature from 1966 to 2000 showed causative effects of mutans streptococci and the lactobacilli for human dental caries, whereas others like sanguinis streptococci, Streptococcus salivarius, or enterococci could not be associated with the disease.145 Recent understanding in the caries process has adopted the importance of the homeostatic balance in biofilm, which describes the microbial ecosystem on tooth surfaces, rather than the virulence of individual bacterial species.141 Regardless, pulpal infection is polymicrobial and often starts from incipient caries causing localized pulpal inflammation or pulpitis.
Local invasion of the cariogenic bacteria or a shift in the bacterial content of biofilm can lead to inflammatory changes in the dental pulp. This frequently happens in the absence of caries extension into the pulp chamber. Bacterial by-products relevant to pulpitis include lactic acid, ammonia, urea, lipopolysaccharide (LPS), and lipoteichoic acid (LTA). It is notable that the dental pulp is capable of managing numerous microbial insults because of its extensive intrapulpal lymphatic system. However, an overwhelming pulpal inflammatory response may be induced through various mechanisms and numerous microbial challenges. LPS and LTA bind toll-like receptors (TLRs). These are present on the surface of some immune cells in the pulp, and induce the release of inflammatory mediators such as prostaglandins, cytokines, and chemokines.64 In particular, tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-8, IL-12, and chemokines CCL2 and CXCL2 are well described for their role in pulpitis.52 IL-1 is known to be released from macrophages after stimulation with LPS and responsible for bone resorption leading to apical periodontitis.59 During acute pulpitis, the inflammatory mediators trigger vasodilation, transient increase of pulpal blood flow (PBF), inflammatory cell infiltration, increased intrapulpal pressure, and finally ischemic necrosis of the pulp (Figure 43-4).
In an acute apical abscess, anaerobic bacteria are predominant over aerobic strains and anaerobes, and microaerophiles were predominant in 82% of the cases studied.72 The most prevalent isolated bacteria are Fusobacterium nucleatum, Parvimonas micra, and Porphyromonas endodontalis.133 Depending on the virulence of the organisms and host resistance, a lesion that has been chronic may exacerbate and become an acute apical abscess. The presence of spirochetes is also well documented in apical, as well as periodontal abscesses and has been studied by various identification techniques.27 Spirochetes most often isolated in root canal infections are Treponema denticola and Treponema maltophilium.66,123 When comparing chronic and acute apical abscesses, Baumgartner found that there was a significantly higher incidence of spirochetes in acute abscesses or cellulitis than in asymptomatic infected root canals. Treponema socranskii was the most frequently encountered species.10
Thermomechanical irritants can induce altered pulpal circulation and pulp tissue damage. Earlier studies demonstrated that thermal changes caused by dental procedures, such as tooth preparation, led to marked diminution of PBF and plasma extravasation, resulting in an inflammatory response.74,120 Extreme heat caused by dry tooth preparation triggers vascular stasis and hemorrhage in the subodontoblastic vascular plexus.105 It was noted that even a small increase in pulpal temperature (5° to 6° C [41° to 42.8°)F] is capable of inducing necrotic changes in the pulp.171 Pulpal necrosis will eventually lead to apical periodontitis. Likewise, chemical irritants impose measurable changes in the pulp status. A recent study showed the cytotoxicity of dental resin material (2-hydroxyethyl methacrylate [HEMA]) on pulp stromal cells through induction of apoptotic cell death.116 Etching the dentin surface with high phosphoric acid content yields deleterious effects on dental pulp.129 Also, bonding resins used as pulp capping materials led to acute pulpitis and varying degrees of necrosis in human teeth.2 Root canal overfills with gutta-percha and sealers invariably cause severe inflammatory reactions in the apical tissues, even though patients may be completely asymptomatic.122 Thus dental materials often contain chemical irritants that affect both the pulpal and periodontal tissues, and one must be cognizant of the potential iatrogenic endodontic pathosis associated with their misuse.
Classification of Pulpal and Apical Diseases
The diagnosis of endodontic lesions that may often have a periodontal component can be confusing. The diagnostic terminology used in various dental schools and textbooks further compounds the confusion for students and practitioners alike. In an attempt to simplify and unify a standardized diagnostic terminology, the American Association of Endodontists established a new endodontic diagnosis terminology in 2009. The reader is referred to this revised terminology published in 2009 in volume 35 of the Journal of Endodontics (page 1634) for a more complete discussion of this subject. Throughout this chapter we will be referring to this new terminology, which is also summarized in Tables 43-1 and 43-2.
TABLE 43-1
Classification of Pulpal Diseases*
| Pulpal State | Symptom | Vitality | Response to Cold |
| Normal pulp | Asymptomatic | Vital | Within normal limits |
| Reversible pulpitis | Sensitive to pressure or temperature† | Vital | Hypersensitive to cold |
| Symptomatic Irreversible pulpitis‡ | Spontaneous, throbbing pain | Vital | Hypersensitive to cold and lingering response |
| Asymptomatic irreversible pulpitis§ | None | Vital | Within normal limits |
| Pulp necrosis¶ | Asymptomatic¶ | Nonvital | No response |
| Previously treated | Variable | Nonvital | No Response |
| Previously initiated therapy | Variable | Variable | Variable |
*Different pulpal conditions cannot be discerned by periapical radiographs. Symptoms and responses to cold explained herein are the general findings, but exceptions can occur.
†Patients’ response to pressure may be due to hyperocclusion in reversible pulpitis cases. In the absence of such factors, patients’ complaints mainly revolve around thermal sensitivity.
‡Symptomatic irreversible pulpitis may be symptomatic and painful as described or may be asymptomatic and nonpainful.
§Asymptomatic irreversible pulpitis is based on presence of pulpal inflammation, e.g., extensive caries, hyperemia, or traumatic injuries, in the absence of patients’ subjective symptoms.
¶Necrotic pulp may cause acute exacerbation of symptom, including spontaneous throbbing pain. However, such sensitivity results from periradicular inflammation.
This diagnostic terminology is based on the recommendations of the AAE Consensus Conference, as shown in the Journal of Endodontics, 35:1634, 2009.
TABLE 43-2
Classification of Periradicular Diseases*
| Periradicular State | Symptom | Pulpal Status | Percussion Response | Palpation Response | PARL | Sinus Tract |
| Normal periapex | None | Varies† | None | None | Not present | Not present |
| Symptomatic apical periodontitis‡ | Painful | Inflamed | Painful | Varies§ | Not present | Not present |
| Asymptomatic apical periodontitis | None | Nonvital | None | None | Present | Not present |
| Acute apical abscess‡ | Painful | Inflamed | Painful | Painful | Varies¶ | Not present |
| Chronic apical abscess | None | Nonvital | None | None | Present | Present |
| Condensing osteitis | None/pain | Inflamed | None | None | Radiopaque | Not present |
PARL, Periapical radiolucency.
*Symptom and other descriptions of the individual periradicular pathosis are the general findings to which deviations can occur.
†Normal periapex can be associated with either normal, inflamed, or necrotic pulp.
‡Difference between acute periradicular periodontitis and acute periradicular abscess is that the former is confined to the involved tooth and the latter is more generalized and frequently presents with gross swelling in the affected periradicular tissues.
§Palpation in acute periradicular periodontitis may elicit tenderness after progression of the disease through the cortical plate.
¶PARL may be present in advanced acute periradicular abscess.
This diagnostic terminology is based on the recommendations of the AAE Consensus Conference, as shown in Journal of Endodontics, 35:1634, 2009.
Previous studies have found little correlation between the histology of pulpal disease and the symptoms that the patient experienced before treatment.84,154 More recently, a review of the diagnosis of pulpal pain by Bender found that 80% of patients who give a previous history of pain manifested histopathologic evidence of chronic partial pulpitis with partial necrosis. This symptomatology would indicate that either endodontic therapy or extraction would be the indicated treatment choices. Bender concluded that a clinician is able to determine the degree of pulp histopathosis by asking patients about their previous pain history and symptoms related to the involved tooth.12 There still remains controversy, however, as to the degree of correlation between pulpal symptoms and the histopathology of the pulpal tissues and additional studies are needed to confirm the correlation between the two.
Apical diseases of endodontic origin are inflammatory processes occurring in the tissues that surround the tooth. They result from various infectious agents that originate in the root canal system and create a series of both inflammatory and immunologic responses. These agents exit through the apical foramen, lateral canals, or dentinal tubules.76,117 It is an infectious process caused by a large number of microbial species unlike classic infectious diseases occurring elsewhere in the body that may consist of only one or two specific organisms. These species reside in ecologically balanced communities discussed previously and referred to as biofilms.114 The different characteristics of pulpal and periodontal lesions are summarized in Table 43-3.43
TABLE 43-3
Different Characteristics of Pulpal and Periodontal Lesions*
| Primary Pulpal | Primary Periodontal | Independent Endodontic-Periodontic | Combined Endodontic-Periodontic | |
| Patient symptom | Varies† | Mild discomfort | Varies† | Varies† |
| Coronal integrity | Compromised | Intact | Compromised | Compromised |
| Radiographic lesions | PARL | Crestal bone loss | Separate PARL and crestal lesions | Continuous bony lesions from alveolar crest to apex |
| Vitality | Nonvital | Vital | Nonvital | Nonvital |
| Periodontal probing | Narrow probing to apex‡ | Generalized bone loss | Generalized bone loss | Generalized bone loss with narrow probing to apex |
PARL, Periapical radiolucency.
*These are generalized summaries, and deviations can occur.
†Patient symptom for pulpal lesions may vary, depending on the type of pathosis. Chronic lesions can be completely asymptomatic, whereas acute symptoms without any radiographic lesions can trigger pain.
‡Primary pulpal lesions may not present with any periodontal defect. Narrow probing in pulpal lesions may indicate sinus tract through sulcus.
Biologic Effects of Pulpal Infection on Periodontal Tissues
When the pulp becomes necrotic, however, it produces a significant inflammatory response involving extremely complex inflammatory and immune reactions. This response can traverse the apical foramen, the furcation, lateral canals, dentinal tubules, or areas of trapped necrotic tissue along the surface of the root that extend past the periodontal ligament (PDL) and into the surrounding apical tissues.130 This initial inflammatory response of the pulp and subsequent necrosis that permeates through the numerous spaces of the canal system includes various bacterial strains, spirochetes, fungi, yeasts, and viruses.107 The nature and extent of the periodontal destruction that follows depends on the virulence of the pathogens in the canal system, the duration of the disease, and the defense mechanisms of the host.20
In a classic study by Kakehashi et al,68 the infected pulps of germ-free rats remained vital, whereas the infected pulps of normal rats that were left open to the oral environment developed pulpal necrosis with subsequent inflammation and formation of periapical lesions. Other investigators reported similar results using other animal models.102
Bacteria play a critical role in both endodontic and periodontal disease. Most bacteria grow in biofilms. The biofilm is composed of a 15% cellular component and a matrix material that comprises the remaining 85%. The formation of biofilm communities is under the control of complex chemical signals that both regulate and guide the formation of the slime-enclosed colonies and water channels.24 Proteolytic bacteria predominate in early root canal infections and then change over time to a flora that contains a greater number of anerobes.34,138
Fungi and yeasts are also present in pulpal infections.158 Studies by various investigators report that the incidence of cultured samples of fungi and yeasts from untreated root canals vary from 0.5% to 26%,125 whereas the percentage in teeth that have been previously endodontically treated showed an even greater increase of these organisms. Candida albicans was the most prevalent isolated species.159
Current evidence suggests the important role that viruses may play in the pathogenesis of both periodontal and endodontic disease. Viruses have been isolated from both patients with periodontal disease and from the dental pulp.22,45 A study by Contreras et al23 demonstrated that gingival herpes viruses were associated with the increased growth of pathogenic periodontal bacteria. Even with the numerous studies that have been published, additional research is needed to demonstrate the causal relationship between viral infections in both periodontal and pulpal disease.125
Several nonliving pathogens have also been implicated in the inflammatory process as well. These include foreign bodies, epithelial rests, cholesterol crystals, Russell bodies, Rushton hyaline bodies, and Charcot-Leyden crystals. These nonliving pathogens have not only been implicated in the inflammatory process but may also be responsible for the lack of healing of apical lesions in teeth that have received appropriate endodontic treatment.125 If the growth of the epithelial cells is stimulated by any of these living or nonliving pathogens, then the integrity of the periodontal tissues may be affected as well.
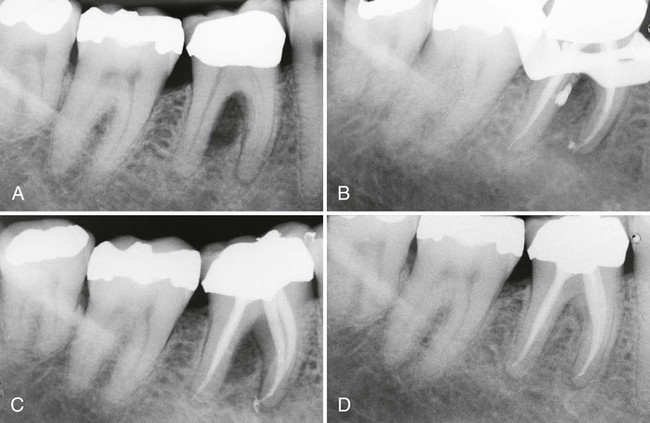
In a few situations, adjacent teeth, their root surfaces, or furcation areas may also probe deeply. Care must be taken to thoroughly test all maxillary and mandibular teeth to correctly assess whether the problem is endodontic or periodontal. Once the correct diagnosis is made, only then should the treatment plan be formulated and discussed with the patient. When endodontic therapy is the main cause of the swelling or breakdown of the periodontium, successful endodontic treatment usually results in healing of both the periapical and periodontal tissues. There are times, however, when trauma to the tooth, severe loss of adjacent periodontal tissues, continued tooth mobility, and occlusal trauma do not provide an environment that allows for apical healing to occur. In these cases, splinting is sometimes necessary to help stabilize the tooth and allow for potential repair of the apical tissues (Figure 43-5).
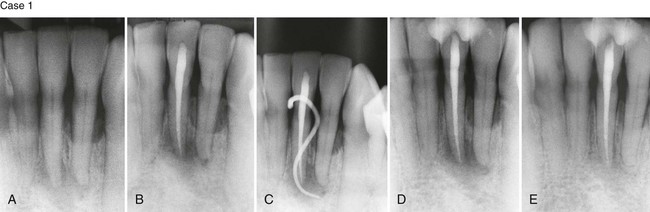
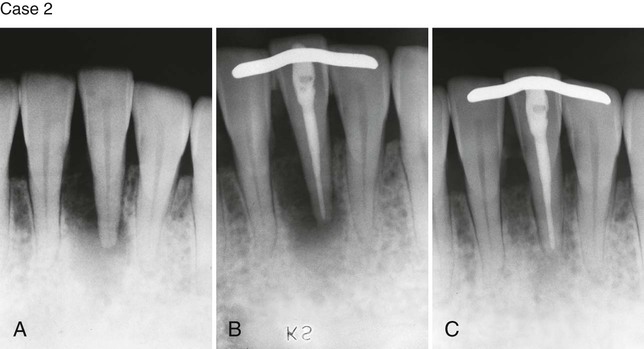
Case 2. A, Previously traumatized tooth #25. The tooth was Class III mobile and tested nonvital to both CO2 and electric pulp testing. B, After obturation of the tooth with gutta-percha, a cast gold splint was bonded to the lingual surface to stabilize the tooth. C, Thirteen-month recall demonstrates repair of the periradicular bone and no mobility as a result of the placement of stabilization and splint. (Case 1 courtesy Dr. Thomas Rauth.)
If an endodontic infection is left untreated, the progression of periodontal disease continues. Untreated and unresolved infections of endodontic origin can sustain the growth of various endodontic pathogens that may lead to increased pocket formation, and bone loss, calculus deposition, osteoclastic activity, and subsequent bone and tooth resorption. They may additionally impair wound healing and aggravate the development and progression of the periodontal disease state.31
The ability of the periodontium to regenerate and heal the lost attachment apparatus has been controversial. This is especially true when the teeth have been endodontically treated and the cement layer is no longer present.71 A study by Sanders et al127 demonstrated a 60% osseous regeneration rate in teeth that had not undergone endodontic treatment compared with a regeneration rate of only 33% in teeth that had endodontic treatment completed. One study compared the loss of attached gingival tissue and found that there was a 0.2 mm greater loss of attached tissue in the presence of teeth with a root canal infection and a periapical radiolucency.62 These same investigators in a later study found a three times greater loss of marginal proximal bone using radiographic measurements in teeth with endodontic infections compared to those without endodontic infections or subsiding endodontic involvement.61 Other investigators, however, have reported that all periodontal tissues have the ability to regenerate, regardless of whether the tooth is vital, partially treated and medicated, partially filled, or whether endodontic treatment has been successfully completed.30 Additional research needs to be completed to better understand the relationship between the presence of endodontic infection and the increased loss of marginal bone and attached tissue in patients prone to periodontal disease.
Biologic Effects of Periodontal Infection on the Dental Pulp
The effects of periodontal disease on the dental pulp appear to be more controversial compared to the effects of pulpal disease on the periodontium.11,131 Not all researchers agree about the effect of periodontal disease on the pulp. Even though inflammation and localized pulpal necrosis have been observed next to lateral canals exposed by periodontal disease,126,130,131 other research studies have not confirmed a correlation between periodontal disease and changes within the pulp.25,97,148 Langeland et al84 indicated that when pathologic changes do occur in the pulp of a tooth as a result of advanced periodontal disease, the pulp does not usually undergo degenerative changes as long as the main canal has not been involved. If the vasculature of the pulp remains vital, no inflammatory reaction occurs and there are no symptoms of pulpal pathosis. An animal study conducted by Bergenholtz14 found that 70% of animal specimens showed no pathologic changes even when 30% to 40% of the periodontal attachment was lost. The remainder showed only minor inflammatory changes, formation of reparative dentin, or resorptive defects where the root had been exposed.14
Researchers and clinicians, however, have observed the spread of advanced periodontal lesions that extend to the apical foramen and result in pulpal necrosis. This retrograde infection may proliferate through large accessory canals on the lateral surfaces of the tooth, canals positioned closer to the apical foramen, and the area where the main canal exits the tooth apex.126 Kobayashi et al81 compared the microflora from root canals and periodontal pockets of caries-free teeth that were necrotic and tested nonvital with an electric pulp tester. The aerobic/anaerobic ratio in the periodontal pocket was 0.23 compared to 0.0022 in the root canal. Although there were far fewer bacteria in the root canal, both areas demonstrated similar bacterial strains. The authors concluded that the similarity of strains in both areas suggested that the periodontal pocket may be the source of bacteria found in infections within the root canal system.79
Protection and preservation of the cementum and dentin surrounding the tooth also play important roles in preserving the health of the pulp and prevent the ingress of periodontal pathogens. The presence of an intact layer of cementum is important in protecting the pulp from dental plaque and other periodontal pathogens that migrate along the root surface during the development of advanced periodontal disease. Excessive root planing and curettage that remove the cementum and dentin from the root surface, encourages narrowing of the pulp canals. This process is thought to be reparative rather than inflammatory.11,85 Several studies also suggest that periodontal disease is degenerative to pulpal tissues resulting in continued calcification, fibrosis, collagen resorption and inflammation.84,94
Dentin thickness also contributes to the protection of the pulp. Stanley137 stated that if a 2-mm thickness of dentin remains between the pulp and irritating stimulus, there is little chance of pulpal damage.137 Weine164 summarized the precautions that can be taken during the course of periodontal therapy as (1) avoid using irritating chemicals on the root surface, (2) minimize the use of ultrasonic scalers when there is less than 2 mm of remaining dentin, and (3) allow minor pulpal irritations to subside before completing additional procedures.164 When these precautions are not followed and the microvasculature of the pulp is damaged during periodontal procedures that involve deep curettage or periodontal surgical efforts to save the tooth, necrosis may result.165
The healing success and failure rates after endodontic microsurgery were studied in teeth that had lesions of only endodontic origin compared to those teeth that had lesions of a combined endodontic-periodontal origin. Lesions only of endodontic origin had a success outcome of 95.2%, whereas those teeth with combined lesions had a success outcome of only 77.5%. This suggests that bone and tissue healing are negatively affected after endodontic surgery with lesions of combined origin.73
It appears, therefore, that both the pulp and periodontal compartments influence the other. Periodontal disease, however, seems to have less of an influence on the pulpal tissues compared to the influence of pulpal disease on the periodontium. Clearly, advanced periodontal disease has some effect on the pulpal state (Figure 43-6). Unless the microvasculature of the pulp is compromised during aggressive periodontal procedures or excessively deep curettage severs the apical vessels, most periodontal interventions result in only a localized pulpal response and dentin hypersensitivity.155
Differential Diagnosis of Pulpal and Periodontal Infection
When the pulpal and periodontal abscesses are separate from one another, most clinicians feel that the diagnosis is usually easier. There are instances, however, when each primary disease may have similar clinical characteristics and make the diagnosis more difficult. In other situations, there may be no demarcation between the two areas of pathosis that both clinically and radiographically appear as one large and continuous lesion with extreme pain and swelling. When this occurs, the clinician must avoid classifying these continuous lesions as true combined lesions. One must rely on all available clinical testing methods to help clarify the correct clinical diagnosis prior to beginning treatment.71
Making the accurate differentiation between pulpal and periodontal lesions can be challenging. If the lesion originates from a pulpal infection and yet was treated by extensive periodontal therapy, it will not resolve. Conversely, performing endodontic therapy on a tooth that has an extensive periodontal defect and a vital pulp will result in the persistence of the periodontal infection. Thus, identifying the primary cause of infection is a critical determinant of treatment outcome. Perhaps the most important consideration when making such a distinction is to base the diagnosis on multiple findings. Those include patients’ symptoms, coronal integrity, shape and size of the radiographic lesions, periodontal probing, and tooth vitality. It is possible that one or more of these findings suggest a pulpal or periodontal infection, whereas others point to the contrary. For example, a tooth may exhibit extensive failing restorations, recurring decay, and radiographic lesions, suggesting probable pulpal involvement. However, the tooth may test completely vital and lack any evidence of an irreversible pulpitis upon thermal testing. Under these circumstances, one would rule out the primary pulpal infection and examine the patient for periodontal involvement. Thus, making the distinction between pulpal or periodontal infection requires collectively dissecting the multiple findings and synthesizing the most probable diagnosis (Table 43-3).
Patients’ Subjective Symptoms
In apical and periodontal abscesses, the extent of pain may vary. In general, an acute apical abscess causes extreme pain to pressure, bite, percussion and, at times, to palpation if the infection has penetrated the cortical bone. Periodontal abscesses are thought to cause less pain because there is little or no elevation of the periosteum. Edema and swelling are characteristics that may be shared by both conditions. The swelling and edema with a periodontal abscess is generally confined to the cervical portion of the tooth. An apical abscess is usually more sensitive to palpation around the tooth apex if the infection has penetrated through the bony cortical plate. Redness and a smooth appearance of the marginal gingival tissues is more common with abscesses of periodontal origin, whereas redness can be detected more apically if a pulpal abscess has started to swell and elevate surrounding tissues. Objective findings in periodontal abscesses include bleeding on probing, suppuration, increased pocket depth, increased tooth mobility, and occasionally, lymphadenopathy.57 Abscesses of endodontic origin usually probe normally but may also display increased mobility, depending on the amount of bone loss. Patients may describe the tooth as feeling longer or higher than the adjacent teeth.48
Suppuration and drainage from periradicular and periodontal abscesses may also differ. Periodontal abscesses are associated with severe periodontal destruction. In a study investigating the incidence of anaerobes in periodontal abscesses, approximately 66% were determined to have a suppurative exudate that was evident during probing. In the same study, 100% of the patients had bleeding during probing, more than 75% had severe edema, redness and swelling, and 78% of the patients had some degree of mobility. Only a few of the patients suffered from lymphadenopathy. Over 60% of the patients had not received previous periodontal therapy and nearly 70% of the teeth were molars. The average measured pocket depth was 7.28 mm.57 In another study that cultured facultative anaerobic bacteria, the teeth most affected were maxillary and mandibular anteriors and mandibular molars. One of the typical features in this study, as in the previous one, was the fact that most of the teeth had been previously untreated.96 Other aspects of periodontal therapy that correlate highly with the devel/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


