CHAPTER 37 Steroid Hormones of Reproduction and Sexual Development*
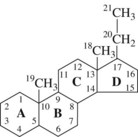
Steroid hormones are derivatives of cholesterol and consist of a combination of three rings of six carbon atoms each (phenanthrene) and one ring of five carbon atoms (cyclopentane) to form a complex hydrogenated cyclopentanoperhydrophenanthrene ring system (Figure 37-1). Steroid hormones can be divided further into three principal sets: corticosteroid hormones (glucocorticoids and mineralocorticoids), Ca++-regulating steroid hormones (vitamin D and its metabolites), and gonadal or sex steroid hormones (estrogens, androgens, and progestins).
STRUCTURE AND FUNCTION
Androgens
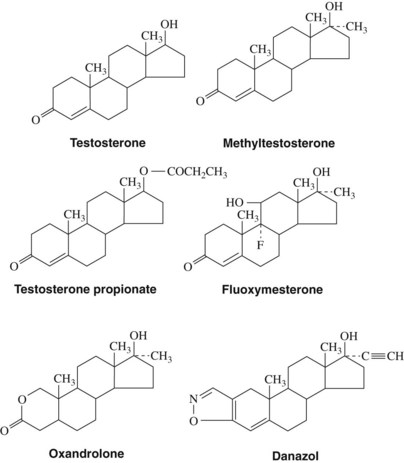
Androgens (Figure 37-2) are derived from a 19-carbon tetracyclic hydrocarbon nucleus known as androstane. One of the most potent androgenic hormones, testosterone, is synthesized by the Leydig cells of the testes, the thecal cells of the ovary, and the adrenal cortex. In men, testosterone is the principal plasma androgen and is reduced to dihydrotestosterone, the mediator of most actions of the hormone.32 The irreversible metabolic conversion of testosterone to dihydrotestosterone occurs only in tissues that contain the enzyme 5α-reductase.51 Testosterone (but not dihydrotestosterone) can also be aromatized to estradiol by numerous extragonadal tissues (primarily adipose tissue and skeletal muscle), a common route of estrogen production in men. In women, the major plasma androgen is androstenedione (androst-4-ene-3,17-dione), which can be secreted into the bloodstream or converted into either testosterone or estradiol by the ovary.
When secreted into the bloodstream, most androgens are transported to their sites of action by a liver-secreted carrier protein termed sex hormone–binding globulin (44% bound) and serum albumins and other proteins (54% bound).7 Secreted plasma androgens are also metabolized to physiologically weak or inactive molecules consisting of either 17-ketosteroids or polar compounds (diols, triols, and conjugates) for excretion by the kidney or liver.20
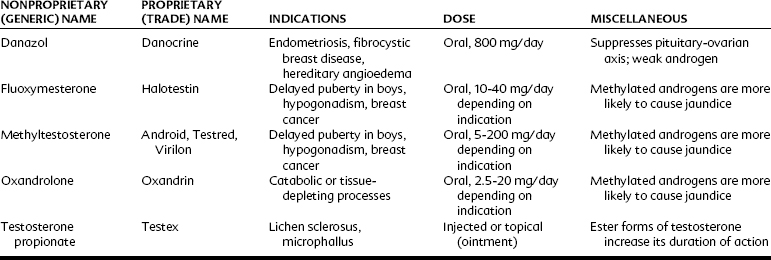
Androgens may be administered orally, topically, or through intramuscular injections (Table 37-1). Testosterone is generally not administered enterally because extensive first-pass hepatic metabolism rapidly reduces plasma concentrations. The bioavailability of androgens is increased by intramuscular injections in an oil vehicle, by transdermal application, or by alkylation at C17, which significantly decreases hepatic metabolism and makes oral administration therapeutically possible.
The biologic activities of androgens are manifested in virtually every tissue of the body. Important functions of androgens include (1) male sexual differentiation of wolffian ducts, external genitalia, and brain in utero; (2) development of adult male phenotype, including growth and maintenance of male sex accessory organs and anabolic actions on skeletal muscle, bone, and hair; (3) facilitation of human sexual behavior; and (4) regulation of specific metabolic processes in the liver, kidney, and salivary glands.32
Estrogens
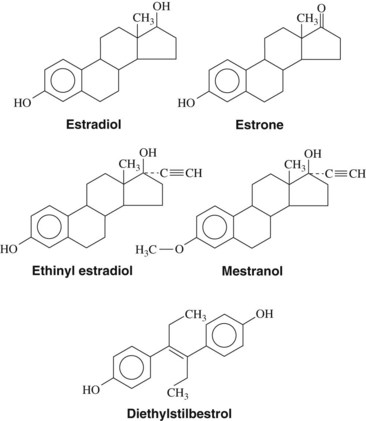
Estrogens (Figure 37-3)—estrone, estradiol, and estriol—are characterized by an aromatic A ring, a hydroxyl group at C3, and either hydroxyl groups (C16 and C17) or a ketone group (C17) on the D ring. Estradiol is the most potent estrogen and is secreted by the ovary, testes, placenta, and peripheral tissues. Estrone is also secreted by the ovary; however, the principal source in women and men is through extragonadal conversion of androstenedione in peripheral tissues.43 In premenopausal women, the most abundant physiologic estrogen is estradiol; in men and postmenopausal women, the most abundant estrogen in the plasma is estrone.54 Similar to other lipid-soluble hormones, estrogens are transported in the blood principally bound to carrier proteins; estradiol in the plasma is bound by either albumin (60%) or sex hormone–binding globulin (38%), leaving only 2% of the hormone free.53 Estradiol and estrone are metabolized principally to estriol, which is the major estrogen detected in the urine.
Estrogens may be administered orally, topically, or through intramuscular injections (Table 37-2). Although estradiol is available for enteral administration, it is generally not used in this manner because concentrations in the bloodstream remain low because of extensive hepatic metabolism.10 The half-life of estrogenic compounds can be increased by synthetic substitutions on the C or D ring (see Figure 37-3). The half-life of estradiol is a few minutes, whereas the half-life of ethinyl estradiol (ethinyl substitution at the C17 position) may be more than 13 hours. Nonsteroidal compounds may also have estrogenic activity; examples of such compounds include diethylstilbestrol, flavones, isoflavones, and certain pesticides (e.g., p,p′-DDT) and plasticizers (e.g., bisphenol A).
Progestins
Progestins (Figure 37-4), or steroids that have progestational activity, are derived from a 21-carbon saturated steroid hydrocarbon known as pregnane. The principal progestational hormone secreted into the bloodstream is progesterone, which is synthesized and secreted by the corpus luteum, placenta, and adrenal cortex. As with androgens, most progesterone is transported in the bloodstream by plasma proteins; however, progesterone in humans is primarily nonspecifically bound to globulin and albumin proteins. The fate of plasma progesterone depends on hepatic, extrahepatic, and extra-adrenal metabolism. 5-α-Dihydroprogesterone and deoxycorticosterone are the most probable active progesterone metabolites. Metabolic inactivation of progesterone to pregnanediol is accomplished by the liver.
Progestins may be administered orally, topically, or through intramuscular injections (Table 37-3). Progesterone is available for enteral administration; however, it is generally not administered in this manner because concentrations in the bloodstream remain low because of extensive first-pass hepatic metabolism. The bioavailability of progestins can be increased by intramuscular injections in oil, by vaginal suppositories, or by ethinyl or other substitution at C17, which significantly decreases hepatic metabolism.
MECHANISM OF ACTION
The current hypothesis of sex steroid hormone action2 begins with the absorption of the hormones into the bloodstream, where they circulate, principally bound (approximately 98%) to plasma proteins. In the circulation, the unbound or free hormone can enter the cell by diffusion and bind to receptors. These large intracellular protein receptors are located in the nucleus of the cell. When the steroid hormone is bound to the receptor, it transforms the receptor to an active configuration, and the activated receptor-steroid hormone complex binds with high affinity to specific nuclear sites (e.g., discrete DNA sequences, nuclear matrix, nonhistone proteins, nuclear membrane). When the receptor-hormone complex is bound to nuclear regulatory elements, a coactivator is usually recruited to the promoter region to allow gene activation and transcription of messenger RNA. After the nuclear interaction, the receptor-hormone complex dissociates, leaving an unoccupied receptor and the steroid hormone. The dissociated receptor is thought to be in an inactive configuration that requires conversion to a form that can bind the steroid again, and the steroid hormone is metabolized and eliminated from the cell.
Although the regulation of gene transcription by hormone-receptor complexes in the nucleus seems to be the major biologic action of sex steroid hormones, these molecules also have other behaviors that are distinct from actions at nuclear receptors. Androgens, estrogens, and progestins have membrane effects and can influence the production of second messenger systems, which can affect neural transmission, the transport of Ca++ ions into cells, and the intracellular concentration of polyamines.28
Steroid Hormone Receptor Structure
The receptors for steroid hormones are able to initiate a wide assortment of responses yet are very similar to one another, not only in their mechanism of action, but also in their structure.9,22 In general, steroid hormone receptors consist of asymmetric protein subunits with long (10 :10 1) axial ratios. These subunits, which form either dimers or tetramers at low ionic strengths, range in weight from 80 to 100 kDa. As a class of regulatory proteins, the different steroid hormone receptors have a high degree of homology. Each protein can be divided into six sections, designated as regions A through F.32
The A/B regions located at the N-terminal are exceedingly variable in size (50 to >500 amino acids) and have negligible amino acid similarities between different receptors. The C region, located between the N-terminus and C-terminus, is a remarkably conserved area that contains the DNA-binding domain. The hydrophilic D region is not conserved in length or sequence but may serve as a hinge between the hormone-binding and DNA-binding domains. The E/F regions located at the C-terminal are similar in size (250 to 300 amino acids), have moderate amino acid homology among the different steroid receptor proteins, and contain the hormone-binding domain. Areas in the N-terminal and the C-terminal are responsible for the transcriptional activation of the DNA.13,23
From these six regions, two important binding domains are present for sex steroid hormone receptors. In one binding domain, the functional activation of the receptor depends on a distinct, high-affinity binding site for a specific hormone. This steroid hormone-binding domain is a large hydrophobic region located near the C-terminal. The other receptor-binding domain recognizes specific sites on DNA. This DNA-binding domain of the steroid receptor is a highly conserved area that contains a tetrahedral arrangement of four cysteine residues around a zinc ion to form a zinc finger-like structure.30 On activation of the receptor, the receptor-steroid complex binds to a specific site on the DNA that is referred to as a steroid responsive element. Steroid responsive elements are unique for each receptor but have common nucleotide characteristics.
THERAPEUTIC USES
Estrogens
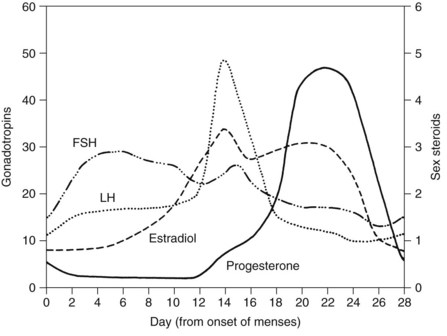
The two principal reasons for the prescription of estrogens are for the prevention of conception and to reduce the sequelae associated with declining hormone levels after menopause. Oral contraceptives are among the most widely used medications in the world and most often are a combination of estrogens and progestins (Table 37-4). Combination oral contraceptives principally affect conception by suppressing the surge of luteinizing hormone, which consequently prevents ovulation (Figure 37-5).24 The estrogen component of combination oral contraceptives usually contains either ethinyl estradiol or mestranol. In these preparations, the estrogen content ranges from 20 to 50 µg; pills containing less than 35 µg are usually considered low-dose contraceptives.
| PROPRIETARY (TRADE) NAME | ESTROGEN | PROGESTIN |
|---|---|---|
| Ovcon-35 (monophasic), Ortho-Novum 10/11 (biphasic), Necon 7/7/7 (triphasic), Loestrin 24 Fe* (monophasic) | Ethinyl estradiol | Norethindrone |
| Norinyl 1/50 (monophasic), Ortho-Novum 1/50 (monophasic) | Mestranol | Norethindrone |
| Ortho-Cyclen (monophasic), Ortho Tri-Cyclen (triphasic) | Ethinyl estradiol | Norgestimate |
| Lo/Ovral (monophasic), Cryselle (monophasic) | Ethinyl estradiol | Norgestrel |
| Yaz* (monophasic), Yasmin (monophasic) | Ethinyl estradiol | Drospirenone |
| Seasonale (84-day therapy, extended cycle), Seasonique (84-day therapy, extended cycle) | Ethinyl estradiol | Levonorgestrel |
| Lybrel† | Ethinyl estradiol | Levonorgestrel |
| Micronor† | — | Norethindrone |
| Depo-Provera | — | Medroxyprogesterone acetate |
| Implanon (implant) | — | Etonogestrel |
| Ortho Evra (transdermal) | Ethinyl estradiol | Norelgestromin |
| NuvaRing (transvaginal) | Ethinyl estradiol | Etonogestrel |
| Plan B (emergency)‡ | — | Levonorgestrel |
| Preven (emergency)‡ | Ethinyl estradiol | Levonorgestrel |
* 24-day formulation followed by 4 days of inert tablets.
† Continuous therapy, without a placebo or pill-free period.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses