CHAPTER 36 Insulin, Oral Hypoglycemics, and Glucagon
INSULIN AND THE ENDOCRINE PANCREAS
The pancreas has exocrine and endocrine functions. The exocrine system comprises the acinar cells, which secrete digestive enzymes. The islets of Langerhans, which make up the endocrine system, contain four types of cells, each of which synthesizes and secretes different polypeptide hormones (Table 36-1). Insulin is produced by the β cells, which constitute most (60% to 80%) of the islet and form its central core. The β cell is the primary glucose sensor for the islet.
| CELL TYPE | HORMONE SECRETED |
|---|---|
| α (A) cell | Glucagon |
| β (B) cell | Insulin, amylin (islet amyloid polypeptide) |
| δ (D) cell | Somatostatin |
| F (PP) cell | Pancreatic polypeptide |
| G cell | Gastrin |
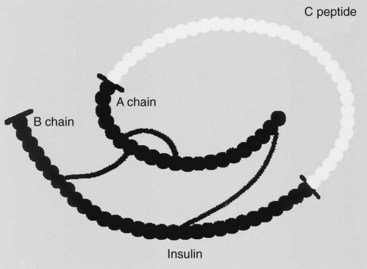
Insulin is a polypeptide containing 51 amino acids. It has a molecular weight of approximately 5800 Da. It is composed of two chains (called the A and B chains) that are joined by two disulfide bridges. Insulin is formed by proteolysis of a large, single-chain precursor, proinsulin. In proinsulin, shown in Figure 36-1, the A and B chains are joined by a connecting (C) peptide. Proinsulin is converted to insulin when the C peptide is removed; this occurs within the secretory granules of the pancreatic β cell. Approximately equimolar amounts of insulin and C peptide are stored in the granules and released by exocytosis when the β cell is stimulated. C peptide has no known biologic function, but it can serve as an index of insulin secretion. Units of insulin, originally defined by activity, are now defined on the basis of weight. There are approximately 28 U/mg of insulin.
Regulation of Insulin Secretion
The pancreas secretes insulin into the portal vein. Insulin secretion is a tightly regulated process designed to provide stable concentrations of glucose in the blood during fasting and feeding. Regulation of plasma glucose is achieved by the coordinated interplay of various nutrients, gastrointestinal hormones, pancreatic hormones, and autonomic neurotransmitters. A basal secretion of insulin is present during fasting periods.15 There is a subsequent rapid increase in insulin secretion after ingestion of a meal. Glucose is the principal stimulus to insulin secretion in humans. It is more effective in provoking insulin secretion when taken orally than when administered intravenously.5
Actions of Insulin
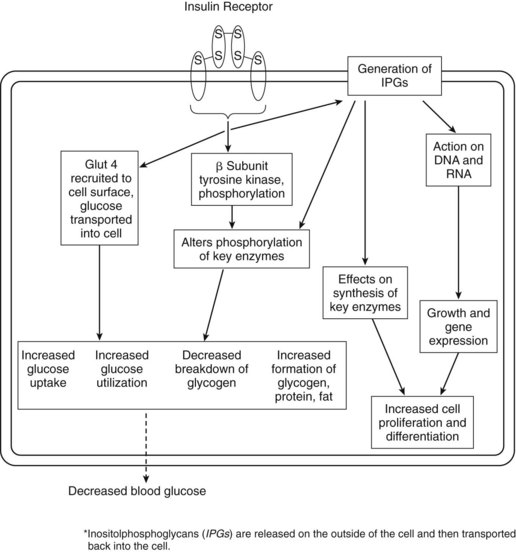
The classic action of insulin is to decrease the blood glucose concentration. Insulin does this by affecting glucose use and glucose production. Liver, muscle, and fat are the important target tissues for regulation of glucose homeostasis by insulin, but insulin exerts potent regulatory effects on other cell types as well. Insulin stimulates glucose transport into muscle and fat by promoting translocation of the intracellular transporter, glucose transporter 4 (Glut 4), to the cell surface (Figure 36-2).14 Insulin does not stimulate glucose uptake into the liver, but it inhibits hepatic glucose production. Insulin inhibits catabolic processes, such as breakdown of glycogen, fat, and protein. Glycogenolysis and gluconeogenesis are inhibited. Insulin receptors are found on virtually all cells. Activation of the insulin receptor leads to a cascade of phosphorylation or dephosphorylation reactions, or both. As a result, insulin affects the activities of various enzymes involved in intracellular use and storage of glucose, amino acids, and fatty acids. Glycolysis (use) and glycogen synthesis (storage) are promoted. The effects of insulin are summarized in Table 36-2.
| TYPE OF METABOLISM | ACTION OF INSULIN | MAJOR TARGET TISSUE* |
|---|---|---|
| Carbohydrate | Increases glucose transport | Muscle, fat |
| Increases glycogen synthesis | Liver, muscle | |
| Decreases gluconeogenesis | Liver | |
| Increases glycolysis | Liver, muscle | |
| Increases glucose oxidation | Fat | |
| Fat | Increases lipogenesis | Liver, fat |
| Decreases lipolysis | Liver, fat | |
| Increases synthesis of triglycerides | Fat | |
| Protein | Decreases protein breakdown | Liver |
| Increases protein synthesis | Muscle, various | |
| Increases amino acid uptake | Muscle, various |
* Insulin exerts potent regulatory effects on other cell types in addition to liver, muscle, and fat, the classically important target tissues for glucose regulation.
In addition to the short-term metabolic effects, insulin has other, longer term actions. It affects synthesis of key enzymes and is believed to have important growth-regulating effects in vivo. Insulin regulates gene transcription,13 affecting protein synthesis; increases cell proliferation and differentiation; and decreases apoptosis.
Insulin Receptor Interactions
The insulin receptor in mammalian cells is a large transmembrane glycoprotein. It is composed of two α subunits and two β subunits linked by disulfide bonds to form a β-α-α-β heterotetramer. Binding of hormone to the α subunits of the insulin receptor leads to the rapid intramolecular autophosphorylation of tyrosine residues in the β subunits. A series of events is initiated that culminates in a cascade of phosphorylation or dephosphorylation reactions. This activity is shown schematically in Figure 36-2.
Insulin Signaling
There is evidence that insulin acts by synthesis of second messengers that enter the cell to mediate some of the hormone’s actions on intracellular enzymes (e.g., phosphorylation, dephosphorylation). These mediators are of the inositolphosphoglycan (IPG) class.8 IPGs represent a family of second messengers or mediators that are increasingly being implicated as having an important role in signal transduction, not only for insulin, but also for other hormones and growth factors. They are discussed later in this chapter.
DIABETES MELLITUS
Evidence indicates that the incidence of type 1 and type 2 diabetes mellitus is increasing worldwide. In 1999, the prevalence was predicted to double by 2010.7 Type 2 diabetes is becoming increasingly common and is an emerging problem in children and adolescents, particularly minorities.3 In the United States, the annual number of newly diagnosed diabetes cases tripled during 1980-2005. Major risk factors for type 2 diabetes are obesity and physical inactivity. The incidence of type 1 diabetes is reported to be increasing by approximately 3% per year.16
Type 1 Diabetes Mellitus
There is considerable evidence that type 1 diabetes is an autoimmune disease of the pancreatic β cell, resulting in degeneration. In type 1 diabetes, there is an absolute lack of insulin. Genetic predisposition and environmental components are involved, with the incidence in homozygous twins being approximately 50%.17 Approximately 5% to 10% of diabetics have type 1 diabetes.
Type 2 Diabetes Mellitus
Approximately 90% to 95% of diabetics have type 2 diabetes mellitus. In type 2 diabetes, target cells are relatively insensitive to insulin.6 This is known as peripheral resistance to insulin. Impaired glucose metabolism in muscle and liver are key features of type 2 diabetes. Genetic predisposition is important in type 2 diabetes; there is greater than 95% concordance in identical twins.17 In addition, most type 2 diabetics are obese. Type 2 diabetics have impaired glucose taste detection,11 which may reflect a generalized defect in glucose sensitivity, including the glucose-sensing pancreatic β cells.
Insulin Therapy
Insulin preparations
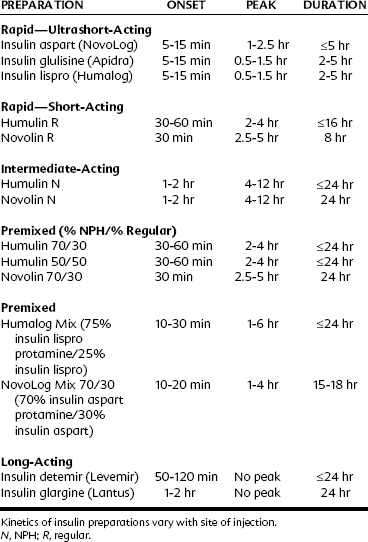
Insulin preparations are classified according to their duration of action into rapid-acting (ultrashort-acting and short-acting), intermediate-acting, and long-acting preparations. Insulin products available in the United States are listed in Table 36-3.
Rapid-acting (ultrashort-acting and short-acting) insulin preparations
Ultrashort-acting insulin preparations—insulin aspart (NovoLog), insulin glulisine (Apidra), and insulin lispro (Humalog)—all are insulin analogues. They may be used with a pump.* Regular insulins (Humulin R and Novolin R) are short-acting preparations. They are soluble, have a rapid onset, and are dispensed as clear solutions at neutral pH.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses