Infection in the Patient with Maxillofacial Trauma
Systemic infections are likely to occur in the traumatized patient through two avenues. First, bacteria may gain direct entry to the host’s systemic circulation through the site of injury, IV line, or urinary catheter. Second, invasive manipulations, such as endotracheal intubation, may bypass previously competent host defense mechanisms, causing pneumonia. Thus, although the maintenance of life is addressed during the initial treatment, early interventions are necessary to prevent potentially disastrous infections. In fact, sepsis is the most frequent cause of death following trauma.1
Causes of Infection
Local Factors
It is known that a sufficiently large number of bacteria are necessary to produce an infection. Studies have shown that an initial inoculum must contain at least 105 bacteria/g of tissue for a clinical infection to occur.2,3 In a traumatic injury, however, far fewer microorganisms may cause infection owing to the presence of devitalized tissue and foreign bodies in the wound.
Normally, surface flora are kept to a minimum by skin appendages that secrete various antimicrobial substances. Sweat gland production of lactic acid, amino acids, uric acids, and ammonia is bacteriostatic.4 Secretory immunoglobulin A (IgA) in the oral mucosa is also an important component of the host defense mechanism for controlling bacterial colonization or overgrowth on the mucosal surfaces. Thus, a break in the skin or mucosal surface will not in itself always provide a large enough inoculum to produce an infection.
Vascularity is also an important local factor in the control of invading organisms. If a wound is compromised by vessel trauma, contusion, or edema, the transport of immunologic host defense products to the site of injury is impaired. The decrease in circulation to the tissue provides a more anaerobic environment, which may permit the growth of certain pathogenic organisms that are inhibited by normal tissue oxygen tension. Local compromise in vascularity is sometimes caused iatrogenically by the injection of epinephrine-containing solutions. For example, a subinfective inoculum of Staphylococcus aureus will occasionally produce an infection if placed into tissues that have been injected with epinephrine-containing solutions.5
Foreign bodies within a wound also permit infection to occur with much lower numbers of organisms. Infection-potentiating factors have been identified in soil. These factors are highly charged anionic particles that interfere with leukocyte functions and inactivate antibiotics.6 Inoculation with only 100 bacteria/g of tissue may cause an infection in the presence of soil. Even the placement of a suture will reduce the number of bacteria necessary to cause infection by a factor of 10,000.7 Thus, vigorous débridement and irrigation are necessary components of wound management, along with the use of the fewest number of sutures acceptable.
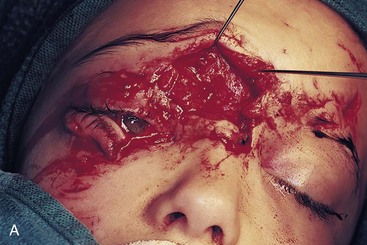
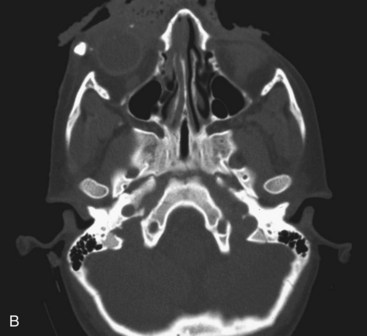
Certain mechanisms of injury are more associated with the presence of foreign bodies within a wound. With a gunshot wound, the missile itself is sterilized on explosion and therefore can be left if imbedded in deeper structures. Tooth or bone fragments may be carried into deeper tissue by the primary or secondary missile and do require removal. Other mechanisms, such as motor vehicle accidents (MVAs), should lead the surgeon to search for broken glass embedded in the wound. Small, puncture-type wounds in these cases may harbor substantial foreign bodies that should be removed during primary wound treatment. These are not often apparent on routine radiographs but may show up on computed tomography (CT) scans (Fig. 32-1).
Irrigation under pressure is recommended to remove foreign bodies and reduce the local concentration of bacteria. Gross et al8 have shown that wounds contaminated with bacteria and sterilized soil were more likely to be rendered sterile if irrigation was accomplished with jet lavage instead of a bulb syringe. The use of large volumes of irrigation will not be effective unless the pressure equals or exceeds 8 psi. Mechanical irrigation devices are available, but the amount of pressure necessary can also be achieved using a 50-mL syringe and a 19-gauge needle. Concerns that the use of a high-pressure irrigation in a wound will further inoculate the site by forcing bacteria into the injured tissue are not well founded. Experimental studies have shown that even with high-pressure irrigation, bacteria and foreign bodies will not be forced more deeply into traumatically injured tissue.9 However, a reduction in particulate material and bacteria within open bone wounds is not improved with the use of high-pressure lavage versus conventional bulb syringes.10
The type of solution used for irrigation is also critical. Further devitalization and tissue damage may occur if toxic solutions are used to irrigate wounds. Studies by Brånemark et al11 have shown that normal saline solution is the least toxic and best tolerated of the commonly used solutions. Solutions such as hydrogen peroxide, chlorhexidine, povidone-iodine, and hexachlorophene cause direct tissue damage. These agents kill fibroblasts and will further devitalize tissue and predispose the wound to infection. “Don’t put in a wound what you wouldn’t put in your eye” is a good approach to irrigating solutions for injuries.12
Systemic Factors
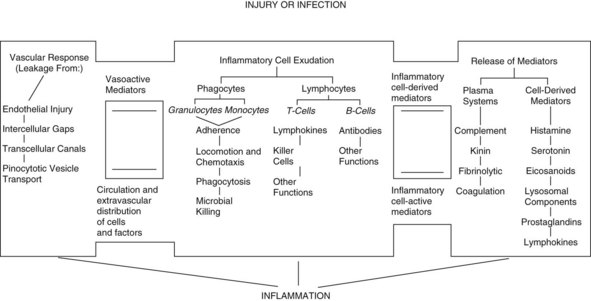
Once the neutrophils have adhered to the endothelial cells, they can be mobilized to the source of infection by following the chemical gradient of chemotactic factors. Some of the known chemotactic factors include various bacterial toxins, the degenerative products of inflamed tissue, and certain reaction products of the complement system and blood clotting systems from the site of injury. The interactions among inflammatory cell exudates, the vascular response to injury, and the release of immunologic mediators are shown in Figure 32-2.
Once the neutrophils are mobilized to the site of the infection, additional reactions are necessary for bacterial killing. First, phagocytosis of the organisms must occur. This phagocytosis is enhanced by the local presence of opsonins, which coat the organism, allowing it to be more readily phagocytosed. Opsonins include the C3b and C5b fragments of the complement system. The phagocytosis of opsonized particles is facilitated in the presence of tuftsin. Tuftsin-releasing enzyme is produced in the spleen and therefore is deficient in splenectomy patients, which increases their risk of infection.13
Anergy
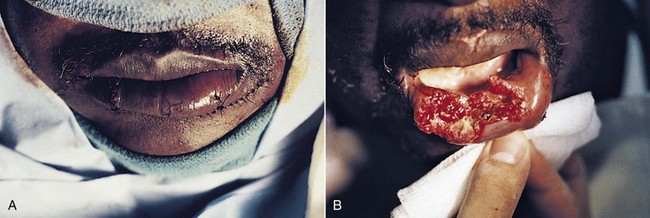
After major trauma, a substantial reduction in immunocompetence may occur. Anergy can often be demonstrated, which shows that a significant loss of the delayed response to infection occurs. Severe trauma also causes overactivation of the T cell suppressor population.14 In addition to these findings, it is known that the stress of trauma increases the output of endogenous epinephrine and corticosteroids. Epinephrine blocks the secretion of insulin, stimulates the release of glucagon and, along with the steroid, increases gluconeogenesis. This combination of events will lead to an abnormal rise in plasma glucose levels and to a notable increase in the susceptibility to infection. The blood glucose level of the patient seen in Figure 32-3 was notably elevated following injury and the persistent hyperglycemia may have led to immunocompromise, wound breakdown, and subsequent infection.
Management of Wounds
On the basis of the history of the injury and direct examination, the surgeon should first evaluate the mechanism of injury to distinguish between blunt and sharp injuries. Significantly more force is necessary to cause soft tissue injury from blunt trauma than from shear forces, such as glass shards or a knife.15 The additional energy absorbed from blunt trauma causes a broader area of tissue contusion, ischemia, and necrosis. Thus, the stellate forehead laceration caused by striking a windshield is far more susceptible to infection than a laceration from a sharp object. The surgeon should consider more aggressive use of débridement and antibiotic prophylaxis in injuries resulting from blunt trauma.
In general, the risk of an infection depends on the following three factors:
• The amount and type of microbial contamination of the wound
• The condition of the wound at the end of the treatment (e.g., the presence of residual necrotic tissue, foreign bodies, and bacterial numbers)
A decision should be reached early about whether primary or delayed closure will be performed. In general, only wounds that are treated early and can be adequately decontaminated should be closed primarily. Because of their rich vascular supply, facial wounds may be closed primarily after a greater delay than would be acceptable in other areas of the body. The risk of infection in facial wounds is reduced because the preinjury quantity of bacteria in the facial region is usually much less than in other areas, such as the foot, in which the numbers and types of bacteria result in a much higher infection rate. Therefore, many authors believe that up to 24 hours following injury is an acceptable period in which to attempt primary closure of facial injuries.17
Wounds of the face are usually closed primarily. Puncture wounds are preferably left open to heal by secondary intention to reduce the potential for infection caused by the trapping of bacteria within the wound. Secondary healing of puncture wounds may also lead to an aesthetically satisfactory scar, especially on a concave surface, such as the medial canthus and nasolabial fold.18 If adequate débridement, irrigation, and principles of closure are followed, this primary closure of facial wounds that are deeper or more extensive provides the most aesthetically satisfying result. More detailed coverage of this topic can be found in Chapter 25. In severely contaminated wounds or those in which a significant delay in treatment has occurred, a delayed primary closure technique should be used. In this technique, the wound is thoroughly débrided, irrigated, and packed open with frequent dressing changes. A wet to dry dressing is applied, which involves moistening sterile gauze in contact with the wound bed and overlaying this with layers of dry gauze. This dressing has a wick effect and draws out serous and any other exudate from the wound. Changing the dressing at least twice daily accomplishes two goals:
1. It permits observation of the wound bed to determine whether an infection is developing.
2. The removal of the pack results in débridement of dead cells and exudate that have adhered to the gauze surface.
The wound is repacked at least twice daily and observed for 3 to 5 days. If no signs of infection are present, the wound margins are sharply incised and primarily closed. Wounds treated by delayed primary closure will heal as fast as those closed primarily, because the reparative processes have already been initiated. It has been shown that as long as a clean wound is closed within 4 days following an incision, the wound strength is equivalent to 7 days, regardless of whether primary closure or delayed primary closure was used.19
Before the closure of questionably contaminated wounds, a technique can be used to provide a rapid estimate of the number of bacteria present in the wound.20 This method may then guide the surgeon in determining whether primary or delayed closure will be used. To perform this test, the wound surface is cleansed with isopropyl alcohol to remove surface organisms and a biopsy specimen is taken from the wound. The specimen is homogenized and diluted 1 : 10 with thioglycolate. With a micropipette, 0.02 mL of the suspension is placed on a glass slide and is confined to an area 15 mm in diameter. The slide is oven-dried for 15 minutes at 75° C (167° F) and then Gram-stained.
The method of wound closure will also affect the chance of infection. As discussed, each additional suture allows an infection to occur with a lesser number of bacteria. However, to prevent the formation of a residual hematoma, sutures must be placed in sufficient numbers to close all the dead space. Studies have shown that approximately one third of all wound infections are due to residual hematoma.21
Hemostasis should be meticulously achieved but not at the expense of creating areas of nonvital tissue in the wound. Careful ligation of vessels and appropriate use of electrocautery should be used . If a hematoma can be predicted because of the exposure of large areas of medullary bone or the raising of a large flap, drainage of the wound should be established.22 A closed system, suction-type drain (e.g., Jackson-Pratt) exiting from a separate stab incision is least likely to serve as a conduit for bacterial ingress into the wound. Drains should be removed as soon as possible, usually within 48 hours or earlier if drainage has ceased.
Topical hemostatic agents are occasionally necessary to arrest bleeding from the cut edges of cancellous bone or injured organs and when the precise source of a continuous ooze cannot be localized. Many formulations are available, including gelatin foam (Gelfoam, Upjohn, Kalamazoo, Mich), microfibrillary collagen (Avitene, MedChem, Humacao, Puerto Rico), and oxidized regenerated cellulose (Surgicel, Johnson & Johnson, Arlington, Tex). The use of these agents must be tempered by the knowledge that most have been shown to act like foreign bodies, predisposing the patient to infection when a normally subinfective inoculum of bacteria is present. Oxidized regenerated cellulose is the only hemostatic agent shown to be bactericidal and thus is the preferred agent.23
For superficial skin closure, reinforced tape (Steri-Strips) has been shown to be superior to a cutaneous suture in terms of preventing infection.24 If skin sutures are placed, they should be removed in 3 to 5 days to preclude tissue reaction, the formation of stitch abscesses, and permanent scarring.
Preparation of the Patient for Surgery
Factors influencing infection in the trauma patient who is scheduled for surgery are as follows:
• Length of the preoperative period of hospitalization
• Use of razors to shave the operative site
• Nature of preparation of the operative site
• Associated resuscitative procedures (allogeneic blood transfusions)
Keeping the preoperative stay short is a factor known to reduce the likelihood of infection by diminishing the period during which colonization with resistant hospital-acquired bacteria may occur. In the traumatized patient, this is accomplished by early operative intervention rather than admitting the patient for a few days before surgery. For example, if there will be a delay in scheduling the operation for an open reduction, the patient could be considered for discharge and then readmitted on the day of surgery. Traumatically injured patients are unlikely to have an infection within 48 to 72 hours of hospitalization, but the rate increases for longer stays.25 Having surgery within 24 hours of admission was shown to reduce the chance of infection when compared with longer time intervals.26
The physical preparation of the surgical patient is also important in controlling the possibility of infection. Although antibacterial agents are most often used in surgical preparation, studies have not shown a decrease in the rate of infection when compared with the rate of infection seen when a simple soap and water scrub is used. This finding is consistent with the fact that it is the mechanical aspect of the surgical scrub that reduces the local factors of infection (e.g., number of bacteria, presence of dirt), and that this mechanical factor is of more value than the antibacterial agent. In an open wound, iodophors and chlorhexidine solutions are contraindicated because they may cause tissue devitalization. A nonionic surfactant (e.g., decylpolyglucose [Sea-Clens, Sween, North Mankato, Minn]) is recommended for cleansing open wounds. This agent will not devitalize tissue and has been shown to be nontoxic, even when injected intravenously. Using the surfactant on a sterile sponge, the wound can be thoroughly scrubbed to remove debris and reduce the amount of bacterial flora. Edlich et al have recommended using this agent exclusively on traumatic wounds.15
Oxygen delivery is critical to the tissue to reduce the potential for infection. The role of oxygen in improving wound healing is being critically reviewed in the literature. Studies have shown that the prediction of infection of a surgical site can be correlated to the local oxygen tension of the wound.27 The administration of supplemental oxygen is therefore postulated to reduce the risk of infection. Studies by Grief28 have demonstrated a reduction in wound infections by the administration of 80% oxygen for the period of surgery and via face mask 2 hours following the completion of the procedure. This easy method of oxygen delivery would indicate a substantial benefit for the patient and consideration for earlier administration to the trauma patient should be considered, particularly if surgery is being deferred.
Because the goal is to deliver this increased oxygen to the tissue, it is the increase in the subcutaneous oxygen tension (PsO2) that is critical to improve bacterial killing by white blood cells. To deliver more oxygen locally, the local tissue perfusion must be optimized. Factors affecting local tissue perfusion include maintenance of normothermia. Because many trauma patients may have prolonged exposure to the elements at the scene of the injury, the surgeon should expect a notable decrease in core temperature on arrival in the emergency department. Peripheral vasoconstriction will reduce local tissue blood flow, reducing the oxygen supply to the injured site. The trauma patient’s core temperature needs to be monitored and consideration for warming blanket placement is usually indicated. Exposure of the patient during the primary, secondary, and tertiary surveys for injuries further reduces the core temperature. Mild hypothermia triples the risk of infection created by the reduction in oxygen supply.29 Finally, even mild hypothermia will increase intraoperative blood loss and the possible necessity for blood transfusions (see later), which are also associated with an increased infection risk.30
Further reducing blood flow to injured tissue is the presence of hypovolemia. Tissue hypoxia is directly related to hypovolemia, so fluid deficits should be corrected. Although not studied in trauma patients, the use of supplemental crystalloids (an increase of 1.1 liters on the first day versus the control group) increased the PsO2 in surgical patients.31 Optimizing tissue perfusion and oxygen delivery involves the administration of greater amounts of crystalloid than what normally would be indicated by observing blood pressure and urine output alone. Fluid supplementation of an initial bolus of 10 mL/kg followed by 16 to 18 mL/kg/hr (continuing for 1 hour postoperatively) notably increases PsO2 in surgical patients.32
One seeming paradoxical issue in the improvement of tissue oxygen supply for reducing infection is that the transfusion of allogeneic blood actually increases the risk of infection. The increase in infection is proportional to the number of units transfused and is postulated to be related to immunosuppression.33 This, in conjunction with hemorrhage, creates an additive immunodepressive effect.34 Of note is that this immunosuppression can be shown to be present for several years with reduced lymphocyte function, natural killer cell cytotoxicity scores, and helper and/or suppressor cell ratios.35
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses