3
Aetiology
Introduction
Biological background
Aetiology of malocclusions in adults
Age-related changes in the skeleton
Age-related changes in the craniofacial skeleton
Age-related changes in the local environment
Intra-age variation is mainly determined by four factors
Consequences of deterioration of the dentition
Case reports
Aggravation of an existing malocclusion
Development of secondary malocclusion
Conclusion
References
Introduction
Adult malocclusions originate from two sources: first, malocclusions that occur during the period of occlusal development, which may worsen with increasing age, and second, as a result of the ongoing, age-related deterioration of the permanent dentition. Marks and Corn (1989) did not differentiate between malocclusions developing during different periods but distinguished between malocclusions of dental and skeletal origin. Among the dental type, they listed intra-arch discrepancies, including malpositions of teeth. Both types of malocclusion can be considered as having both genetic and environmental origins but in the case of the older adult patient, age-related tooth migrations occur as a result of deterioration as well as different types of oral parafunction and dysfunction constitute important aetiological factors.
Biological Background
Understanding of the aetiology of malocclusions requires a background knowledge of the biology related to the oral tissues and surrounding bone. In adult patients, this is even more important as the reaction of the periodontal tissues to orthodontic tooth movement is age related and influenced by both local and general pathological conditions.
The early development of the head and neck structures starts with the branchial arches, which are present from around weeks 5–6 of gestation. These prominences are composed of epithelium, which surrounds mesenchymal cells predominantly of neural crest origin. As the first branchial arch develops to form the mandibular and maxillary processes, the stomatodeum or primitive mouth cavity becomes prominent.
The development of the dentoalveolar region starts in the fourth week when neural crest cells migrate into the branchial arches, forming a band of ectomesenchyme under the epithelium of the primary oral cavity. Interaction between the ectomesenchyme and the epithelium initiates the subsequent formation of the dental lamina from which the tooth buds originate. The tooth bud passes through the cap stage to the bell stage in which the enamel organ, the dental lamina, the dental papilla and the dental follicle can be differentiated (Fig. 3.1). The attachment apparatus with all its components will develop from this follicle. Once the crown has formed, root formation starts. Studies involving transplantation of a tooth germ to a different location clearly indicate that the development of the periodontium is completely dependent on the existence of the dental follicle. When transplanted with its follicle, a tooth bud will give rise to periodontal tissues even in different locations (Kristerson and Andreasen 1984; Palmer and Lumsden 1987) (Fig. 3.2).
Fig. 3.1 Cap stage demonstrating the condensation of ectomesenchymal cells in relation to the dental epithelium, the dental organ (DO), from which the dental papilla (DP) that gives rise to the pulp and the dentine is formed. The dental follicle (DF) is seen surrounding the dental papilla and gives rise to the periodontal tissues.
(Reproduced from Lindhe J, Karring T, Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
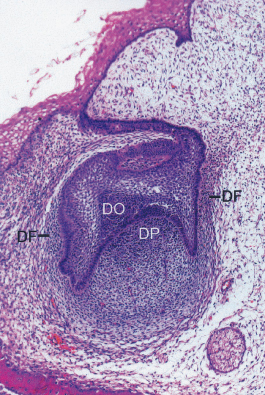
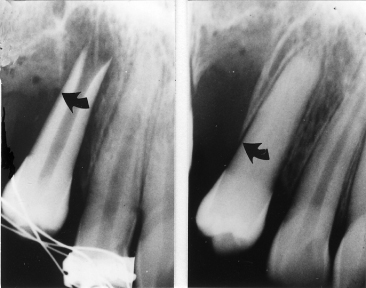
Fig. 3.2 An unerupted premolar transplanted to a canine region with severe loss of bone following an injury. It can be seen that bone formation has been induced during root formation.
(Reproduced from Andreasen JO (1996) Textbook and Color Atlas of Traumatic Injuries to the Teeth, with permission from Wiley-Blackwell.)
The root formation and the formation of the periodontal tissues are closely interdependent and linked in time (Hammarstrom et al. 1996). At the point where the internal and the external dental epithelium cells meet and proliferate in an apical direction, Hertwig’s epithelial root sheath is formed and projects in the future direction of the root (Fig. 3.3). Local pathological processes affect root formation and its shape, length and direction of growth. Developmental anomalies can occur if disturbances occur during any of the stages of tooth development (Table 3.1).
Table 3.1 Development anomalies of the teeth
| Initiation phase | Anodontia |
| Oligodontia | |
| Supernumerary teeth | |
| Bud stage | Microdontia |
| Macrodontia | |
| Cap stage | Dens invaginatus |
| Germination | |
| Fusion | |
| Tubercle formation | |
| Apposition phase hypoplasia | |
| Hypocalcification | |
| Amelogenesis and dentinogenesis imperfecta |
|
| Root formation | Enamel pearls |
| Root dilaceration |
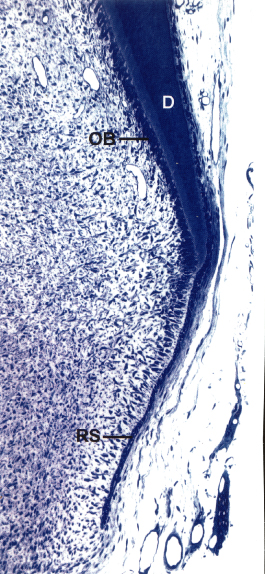
Fig. 3.3 Root development starts when the external and internal dental epithelium meet and proliferate as a double layer of cells, now named Hertwig’s epithelial root sheath (RS). The inner epithelial cells differentiate into odontoblasts (OB) and start forming dentine (D). Formation of dentine continues in an apical direction and generates the framework for the root.
(Courtesy of K. Josephsen, from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
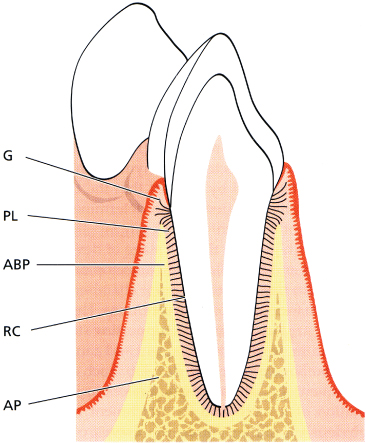
The periodontium, also defined as the attachment apparatus, surrounds the tooth and includes: the gingiva; the periodontal ligament; the cementum; and the alveolar bone (Fig. 3.4). Both the development and the maintenance of the alveolar bone is closely associated with root development and the eruption of the teeth.
Fig. 3.4 Schematic drawing of the normal periodontium: gingiva (G), periodontal ligament (PL), root cementum (RC), alveolar bone (AB), and alveolar bone proper (ABP).
(Reproduced from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
The gingiva is the part of the oral mucosa that covers the alveolar process and surrounds the teeth. The gingiva consists of epithelium covering underlying connective tissue called the lamina propria. The gingiva immediately adjacent to the teeth has been defined as free gingiva and this does not rest on bone (Fig. 3.5). At the free gingival groove (F), which corresponds approximately to the marginal bone level, it continues apically as the attached gingiva (A). The free gingiva does not have the stippled appearance characterizing the attached gingiva. It is pale and smooth and demarcates the cementoenamel junction in young individuals (Fig. 3.5). In older adults, apical displacement of the gingival tissue leads to loss of the free gingiva (Fig. 3.6). The attached gingiva will consequently border the tooth and continue into the most marginal part of the pocket epithelium, the sulcular epithelium. This merges with the junctional epithelium that lines the pocket; it is not keratinized and is only a few cell layers thick (Fig. 3.7). In young individuals the junctional epithelium is attached to the enamel through a laminar structure resembling a basement membrane (Furseth et al. 1986a).
Fig. 3.5 Intraoral view demonstrating the healthy gingiva of a young individual. The gingiva immediately adjacent to the crown of the tooth is smooth and pale pink in colour. It is called the free gingiva (F) since it does not rest on alveolar bone. It is delineated from the attached gingiva by the free gingival groove, which also reflects the underlying cementoenamel junction. The attached gingiva (A) extends from this groove in an apical direction and merges with the free mucosa. The attached gingiva has a stippled appearance due to the small impressions on its surface.
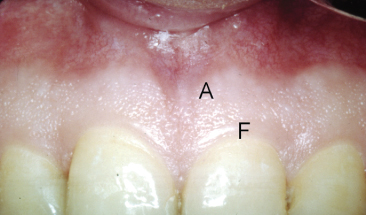
Fig. 3.6 Intraoral image of a 40-year-old woman. The free gingiva has disappeared.
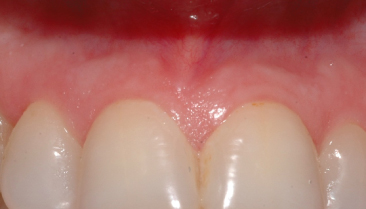
Fig. 3.7 Sagittal histological section through a tooth. It can be seen that the sulcular epithelium comprises only a few cell layers. E, enamel; JE, junctional epithelium; CEJ, cementoenamel junction; OE, oral epithelium; OSE, oral sulcular epithelium.
(Reproduced from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
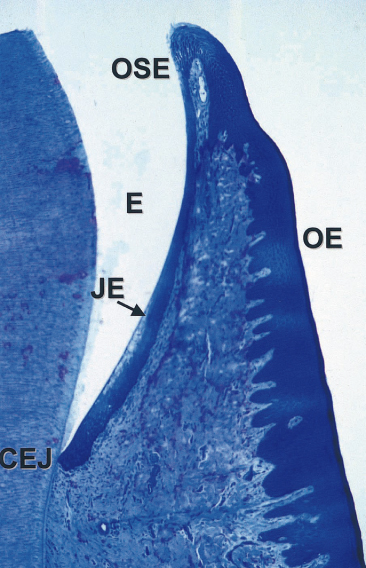
The attached gingiva extends from the gingival margin to the mucogingival junction from which it is sharply delineated by a scalloped line on the buccal aspect (Fig. 3.8). Lingually, there is no sharp demarcation between gingiva and palatal mucosa. The attached gingiva has a typical stippled appearance in young individuals with a healthy gingiva.
Fig. 3.8 (1) Buccal and (2) lingual intraoral views of the alveolar epithelium. On the buccal side, there is a sharp delineation between the keratinized and the non-keratinized gingiva. This is not the case on the lingual aspect.
(Reproduced from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
The gingival epithelium covers the lamina propria, which consists of densely packed collagen fibres, vessels and nerves, and a small component of fibroblasts. According to their function, insertion and attachment, the fibres have been classified as: dentogingival fibres; dentoperiosteal and transseptal fibres (which extend from the cementum of one tooth to the cementum of the adjacent tooth); alveologingival fibres; and the circular fibres circumscribing the tooth. The periodontal ligament is a continuation of the lamina propria and is dominated by collagen fibres that according to orientation and localization are distinguished as alveolar crest fibres, horizontal fibres, oblique fibres and apical fibres (Fig. 3.9). The total fibre system is subject to deterioration in many of the adult patients who present with loss of both periodontal attachment and teeth. The remaining components of the lamina propria comprise the matrix and the cells, predominantly fibroblasts, and also granulocytes, lymphoid cells and mast cells. The distribution of the cells depends on the health status of the gingiva. The function of the matrix is transportation of water, electrolytes, nutrients and metabolites between the cells.
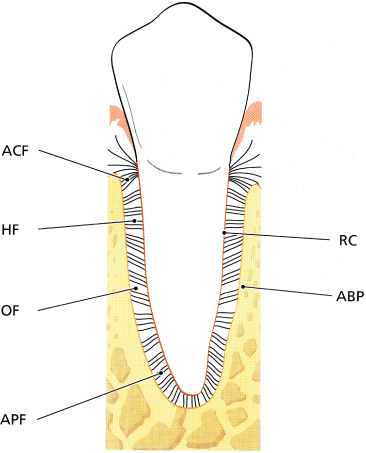
Fig. 3.9 Schematic drawing illustrating how the periodontal ligament is situated between the alveolar bone proper (ABP) and the root centrum (RC). The tooth is joined to the bone by bundles of collagen fibres which can be divided into the following main groups according to their arrangement: ACF, alveolar crest fibres; HF, horizontal fibres; OF, oblique fibres; and APF, apical fibres.
(Reproduced from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
The periodontal ligament connects the teeth with the alveolar bone and is a continuation of the lamina propria. The thickness of the periodontal ligament varies between 0.2 and 0.4 mm depending on its functional status. Also, within the individual tooth there is a variation, as the thickness of the periodontal ligament is minimum at the point around which the tooth tips when submitted to a force system acting on the crown (Fig. 3.10) (Dalstra et al. 2006). The orientation of the collagen fibres in the periodontal ligament varies according to the location of the fibres. The majority are orientated so that they will be stretched when the tooth is loaded with occlusal forces, thereby generating the force system necessary for the maintenance of the alveolar bone (Fig. 3.11). The fibres that extend from the cementum do not always cross through the periodontal ligament but intertwine with those extending from the alveolar bone proper, making up the lamina dura (Fig. 3.12).
Fig. 3.10 Micro-computed tomography (CT) image illustrating the width of the periodontal ligament is smallest in the middle of the root, probably corresponding to the centre of resistance of the tooth.
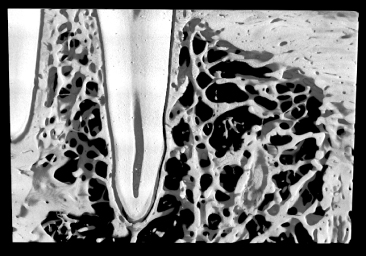
Fig. 3.11 Principal fibres extend from the bone in an apical direction to the cementum of the tooth. The fibres embedded into the cementum have a smaller diameter that the ones embedded into the alveolar wall. PDL, periodontal ligament; ABP, alveolar bone proper.
(Reproduced from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
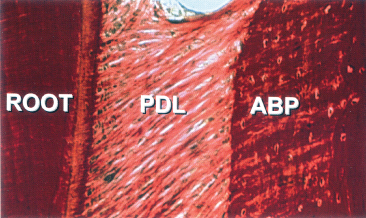
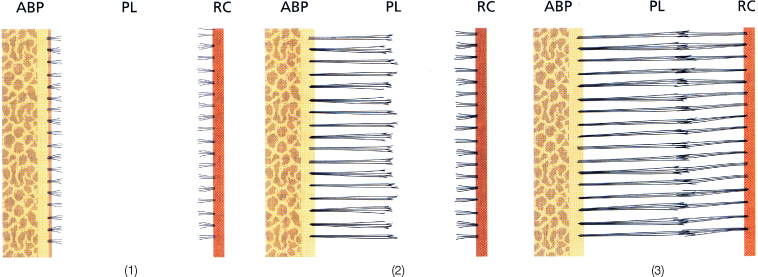
Fig. 3.12 (1–3) Drawing illustrating that periodontal fibres do not extend from the cementum to the alveolar wall and vice versa but become intertwined in the middle of the periodontal ligament (PDL). ABP, alveolar bone proper; RC, root cementum.
(Redrawn from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
The cementum and the dentine covering the root are formed simultaneously as the Hertwig’s sheath is gradually fragmented and the ectomesenchymal cells from the dental follicle differentiate into cementoblasts. The first formed cementum is cellular and attached to the dentine via collagen fibres passing from the dentine. When the thickness of the cementum increases, some cementoblasts are incorporated into what will then become cellular cementum. The cementocytes are interconnected like the osteocytes in bone and also serve the same function as the osteocytes. Formation of the cellular cementum takes place during the functional period of the tooth, it undergoes a certain amount of turnover, for which reason it can also contribute to the repair of minor resorptive areas. A sign of the ongoing turnover is the presence of cutting cones (Williams 1984; Mobers 1991). From this cementum, fibres constituting the essential part of the attachment apparatus extend into the periodontal ligament. This extrinsic fibre system is generated by the fibroblasts of the periodontal ligament, while the internal fibre system, consisting of fibres oriented parallel to the long axis of the tooth, is formed by the cementoblasts. Unlike bone, the turnover of cementum is more like a drift, resulting in gradual increase in thickness of the cellular cementum (Fig. 3.13).
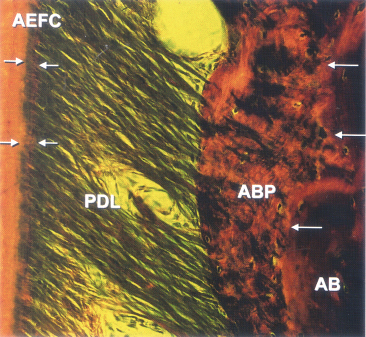
Fig. 3.13 Photomicrograph of a periodontal ligament (PDL) from an area where the root is covered with a cellular extrinsic fibre cementum (AEFC). Arrows indicate fibres inserted into the cementum covering the root and in the alveolar bone proper (ABP) of the other side. The structure of the alveolar bone proper woven bone can be distinguished from the more well-structured alveolar bone (AB) towards the right side of the image.
(Reproduced from Lindhe J, Karring T and Lang NP, eds. (2005) Clinical Periodontology and Implant Dentistry, 4th edn., with permission from Wiley-Blackwell.)
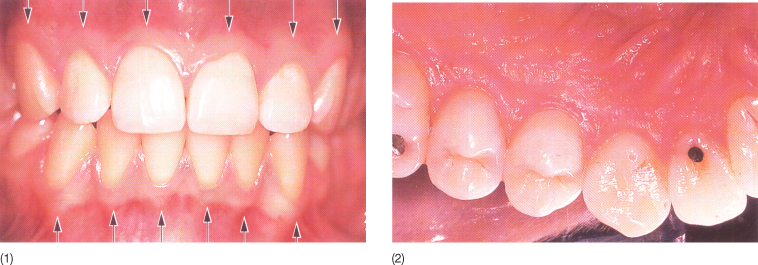
The alveolar bone is, based on its origin, separated into the alveolar bone proper adjacent to the teeth and the surrounding alveolar bone, which consists of trabecular bone and the cortical bone bordering the lingual and buccal aspects of the alveolar process and continuing as the cortical bone covering the basal parts of the jaws (Figs 3.10 and 3.14). The alveolar bone proper has also been termed lamina dura because of its radiographic appearance. However, three-dimensional images clearly show that the lamina dura is heavily perforated and that the term ‘dura’ is a mere projection phenomenon as seen in histological sections (Figs 3.15 and 3.16). The alveolar process including alveolar bone proper and the surrounding trabecular and or cortical bone is entirely dependent on the presence of teeth (Fig. 3.17).
Fig. 3.14 Micro-computed tomography (CT) demonstrating the alveolar bone proper (ABP) as well as the surrounding trabecular bone (T).
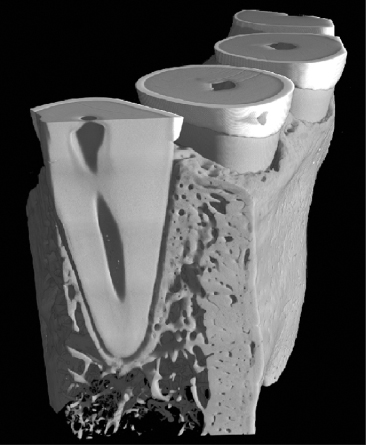
Fig. 3.15 (1) Image of an impression of a dry skull showing an alveolus with a normal bone level and (2) one characterized by marginal bone loss. The fenestrations in the alveolar wall are much more pronounced in the impression of the tooth socket with marginal bone loss, most likely due to the inflammation causing the bone loss.
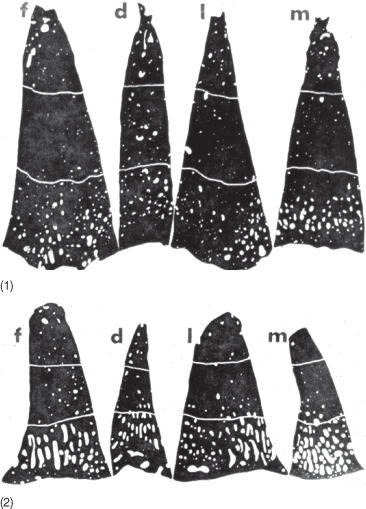
Fig. 3.16 (1) Buccolingual sections of an incisor from a patient with a brachycephalic skull and (2) from an individual with a dolichocephalic skull.
(Courtesy of Birgit Ellegård.)
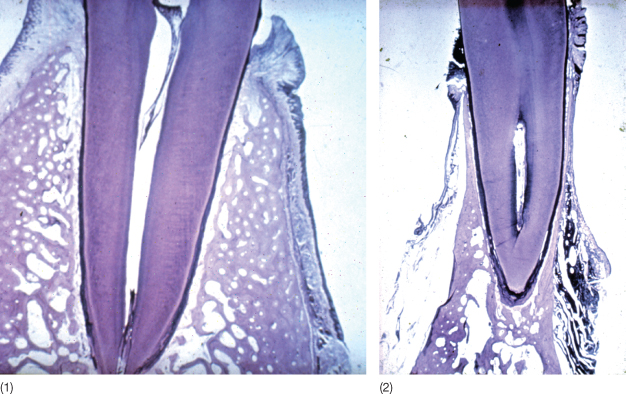
Fig. 3.17 (1) The dependency of the alveolar process on the presence of teeth is clearly demonstrated in this patient with anodontia. (2) Head film of a patient with total agenesis and, as a consequence, absence of the alveolar processes.
(Courtesy of H. Gjoerup.)
The quality and level of the bone reflect the function of the teeth, so that the bone surrounding non-functional teeth will exhibit a reduction in density and develop an inactive osteoporosis. In addition, eruption may occur when no opposing teeth are present (Picton 1964). The marginal bone level is displaced in an apical direction with age (Fig. 3.15). The buccal part of the alveolar bone may be very thin and will with increasing age exhibit both fenestrations or dehiscences. Whereas fenestrations may be related to skeletal morphology, dehiscences are frequently pathological in origin.
The periodontium, as the word indicates, surrounds the teeth and constitutes the tissue delivering support to the tooth. In the case of sufficient support, the teeth exhibit only limited mobility and the thickness of the periodontal ligament is maintained within narrow limits (Lindhe et al. 1997). The level of connective tissue attachment into the cementum of the root determines to a large extent the mobility of the tooth. The marginal bone level is highly, although not uniformly, correlated to the level of attachment.
Aetiology of Malocclusions in Adults
The aetiology of malocclusions presented by adult patients consists of the same genetic and environmental factors as in young patients, but in addition, the ongoing age-related degeneration both general and local contributes to the development of so-called secondary malocclusions.
Analyses of cephalometric variables on radiographs of skulls from various periods have demonstrated that over the past millennia changes have occurred predominantly in the dentoalveolar area and are related to alterations in lifestyle (Carlson 1976; Carlson and Van Gerven 1977). This was also confirmed by Varrela (1992), who studied the development of the craniofacial structures over the past four centuries and by Vyslozil et al. (1996), who compared cephalograms of Austrian soldiers killed in the late eighteenth century with those obtained from soldiers a 100 years later. The differences between the two groups in the latter study were also predominantly dentoalveolar.
Although growth modification in growing individuals is recognized as a legitimate treatment principle, it is generally accepted that the changes achievable in the basal structures are minor and frequently limited to the removal of detrimental environmental influences and dysfunction, thus enhancing the adaptation of the dentoalveolar structures to the existing skeletal basis. In adults, moderate skeletal changes have been generated by the Herbst appliance (Ruf and Pancherz 1999; Paulsen and Karle 2000; Kitai et al. 2002; Ruf and Pancherz 2004). It remains to be shown whether the condylar growth demonstrated is later replaced by dentoalveolar modelling.
Whereas the contribution of hereditary and environmental factors to the malocclusions seen in young patients is not yet fully known and remains under intense discussion, it is evident that the secondary malocclusion observed in the older patients is a product of general and local age-relat/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


