CHAPTER 27 Diuretic Drugs
The reabsorption of water in the kidney is passive, following osmotic gradients created by the movement of solutes along the nephrons to the extent permitted by the water permeability of the various segments of each nephron.10 Water readily equilibrates across the nephron as solute is reabsorbed from the tubular fluid or in response to the medullary osmotic gradient as it descends toward the tip of the loop of Henle. The ascending portion of the nephron is relatively impermeable to water, however.
Antidiuretic hormone (ADH), also known as vasopressin, determines the extent to which this segment is permeable to water.21 If ADH is absent, the tubular fluid that reaches the collecting duct becomes, after some solute exchange, the maximally dilute excreted urine. If the collecting duct is responding maximally to ADH, the most concentrated urine that the kidney can generate is excreted; ADH makes collecting duct cells permeable to water, which permits passive water extraction from the tubular fluid as it passes through the progressively more concentrated osmotic gradient of the medullary interstitium. The more solute that reaches the collecting duct, the greater is the volume of urine for any amount of ADH.
All the drugs discussed in this chapter affect renal function by inhibiting the reabsorptive capacity of the renal nephrons. This action produces an increase in the rate of urine production. Substances that increase the quantity of urine are called diuretics. Many substances produce this effect, including caffeine, alcohol, and water itself; however, most clinically useful diuretics produce their effects by inhibiting Na+ reabsorption by the nephrons.10 Such drugs are properly called natriuretics; even so, in most circumstances, all these drugs are referred to as diuretics. All clinically useful diuretics produce their effects by acting at specific segments of the nephron. Common conditions for which diuretics are used include essential hypertension and congestive heart failure.5,15,17,18
CLASSES OF DIURETICS
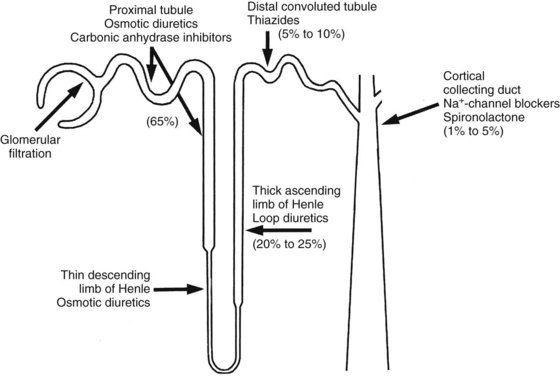
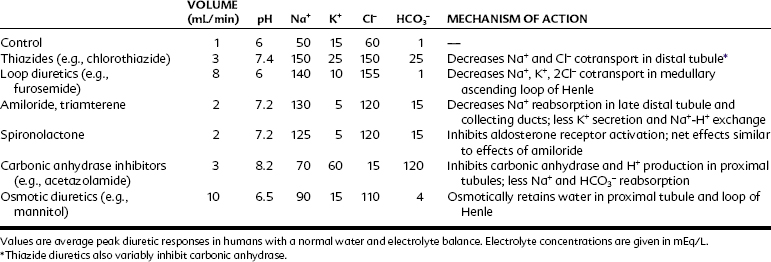
This discussion of diuretics proceeds up the nephron in a retrograde direction. As shown in Figure 27-1, this order of consideration moves from diuretics with a low maximal effect to diuretics with a high maximal effect. The effects of blockade of Na+ reabsorption upstream on tubular fluid composition are acted on by downstream mechanisms—mechanisms that act to limit or offset these effects. The effects of the various classes of diuretics on urine volume, urine pH, and urine electrolytes are summarized in Table 27-1.
K+-Sparing Diuretics
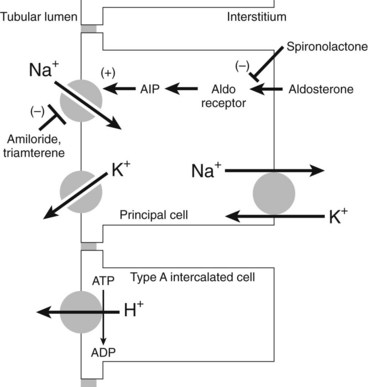
The pathway by which Na+ is reabsorbed in the late distal tubule/cortical collecting duct is shown in Figure 27-2. The apical membrane of these cells contains Na+ channels. The entry of Na+ through these channels carries a net positive charge along with it (i.e., Na+ entry is electrogenic), leaving the lumen with a net negative charge. This negative charge in the lumen acts as a driving force for movement in the opposite direction of other cytosolic cations such as K+ and H+ by the collecting duct (see Figure 27-2), resulting in Na+ retention and K+ excretion. Aldosterone, acting through nuclear receptors in the principal cells of the cortical collecting duct, enhances the conductance of apical Na+ channels. For a given amount of aldosterone, more Na+ delivered to this site means more Na+ reabsorption and more K+ secretion, a coupled process referred to as Na+/K+ exchange.3 Because of the Na+/K+ exchange at this portion of the nephron, any diuretics acting further upstream to block Na+ reabsorption and increase distal Na+ delivery result in enhanced urinary excretion of K+.
Pharmacologic effects
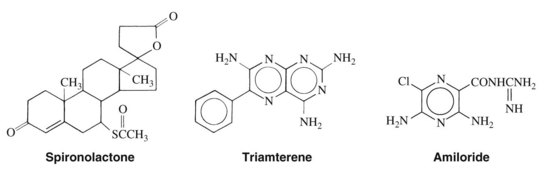
K+-sparing diuretics are so named because, by blocking Na+ reabsorption in the cortical collecting duct region of the nephron, they do not produce the hypokalemic effects of the other natriuretic drugs.20 The three drugs of this class—spironolactone, triamterene, and amiloride—are structurally dissimilar (Figure 27-3), but each produces similar effects (mild natriuresis with a decrease in K+ excretion) because of the blockade of Na+ reabsorption by this pathway.11 Spironolactone is a 17-spirolactone steroid that is structurally similar to aldosterone and functions as an aldosterone antagonist. Triamterene, a pteridine derivative with structural similarities to folic acid, and amiloride, a pyrazine derivative, exert similar effects by directly blocking the apical membrane Na+ channels of the principal cells of the collecting duct. By preventing Na+ entry into these cells, these diuretics reduce the electrogenic driving force for K+ or H+ secretion, or both, in this segment. The net effect is a mild diuresis with a K+-sparing effect.
Therapeutic uses
K+-sparing diuretics are most often used to prevent hypokalemia caused by thiazide and loop diuretics. Spironolactone, triamterene, and amiloride are each available as combination preparations with thiazide diuretics to facilitate this use. Spironolactone is also sometimes used in the treatment of hyperaldosteronism. More recently, spironolactone has been found to be especially useful in the treatment of congestive heart failure (see Chapter 25). Plasma aldosterone concentration is inappropriately elevated in patients with congestive heart failure, and it contributes to the development of edema, direct hypertrophic effects on the myocardium, and other adverse effects in heart failure. Spironolactone effectively antagonizes these effects and has been shown to reduce the mortality rate of patients with congestive heart failure.
Thiazide Diuretics
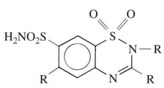
Benzothiazide diuretics (commonly referred to as thiazides) are derived from 1,2,4-benzothiadiazine-7-sulfonamide 1,1 dioxide (Figure 27-4). Chlorothiazide was originally synthesized in an attempt to produce more potent carbonic anhydrase inhibitors. Investigators soon observed that although chlorothiazide produced prompt diuresis as predicted, it did not do so by increasing the excretion of NaHCO3. Rather, it produced a large increase in the excretion of NaCl, suggesting a novel natriuretic and diuretic mechanism: inhibition of Na+-Cl− cotransport in the distal nephron.22 Structural congeners of chlorothiazide, including hydrochlorothiazide, hydroflumethiazide, and methyclothiazide, also share this mechanism. Several other compounds (chlorthalidone, indapamide, metolazone, and quinethazone) that are not structurally related to thiazides also inhibit renal Na+-Cl− cotransport and produce natriuresis and diuresis that is indistinguishable from thiazides. For this reason, it is a common convention to refer to all drugs that inhibit renal Na+-Cl− cotransport as “thiazides” regardless of their structure.
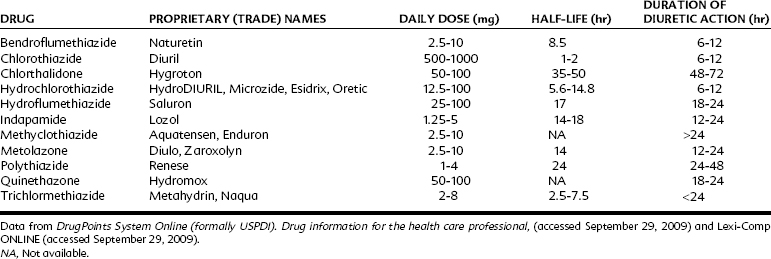
Table 27-2 lists diuretics of the thiazide class available for prescription in the United States. Hydrochlorothiazide is also available in combination form with K+-sparing diuretics (Table 27-3). In addition, there are at least 21 formulations on the market that combine hydrochlorothiazide with another antihypertensive drug.
| PROPRIETARY (TRADE) NAME | THIAZIDE | ADDITIONAL DRUG |
|---|---|---|
| Moduretic | Hydrochlorothiazide 50 mg | Amiloride 5 mg |
| Aldactazide, Spirozide | Hydrochlorothiazide 25 mg | Spironolactone 25 mg |
| Aldactazide | Hydrochlorothiazide 50 mg | Spironolactone 50 mg |
| Maxzide | Hydrochlorothiazide 25 mg | Triamterene 37.5 mg |
| Hydrochlorothiazide 50 mg | Triamterene 75 mg |
Pharmacologic effects
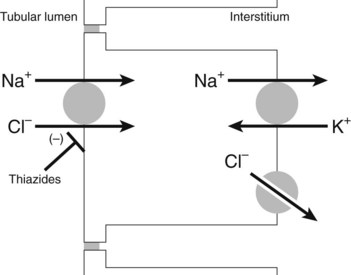
Thiazide and thiazide-like diuretics enter the lumen of the nephron by glomerular filtration and through secretion by the organic acid transporters of the proximal tubule. Thiazide diuretics can achieve a luminal concentration that is higher than their free plasma concentration. Inhibitors of organic acid transport, such as probenecid, can inhibit the action of thiazide diuretics by lowering the luminal concentration. When the drug reaches the distal convoluted tubule, it binds to the Na+-Cl− cotransporter (most likely at the Cl− binding site) and inhibits its turnover (Figure 27-5).17 The result is a reduction in Na+-Cl− reabsorption by the distal convoluted tubule and an increase in the amounts of Na+-Cl− delivered to the cortical collecting duct.22 Some of the Na+ that is delivered to the collecting duct is excreted with an equivalent amount of water, producing natriuresis and diuresis, and some is reabsorbed in the cortical collecting duct as it is exchanged for K+ or H+; thiazides also produce kaliuresis (increased excretion of K+).
At the level of the whole organism, long-term administration of a thiazide diuretic produces a reduction in the extracellular fluid volume.2 The decrease in blood volume activates the renin-angiotensin system, causing angiotensin II–mediated aldosterone release from the adrenal gland. Aldosterone acts on the cortical collecting duct to increase the conductance of the principal cell Na+ channels. Blood volume reduction leads to aldosterone-induced increases in the recovery of Na+ in the cortical collecting duct, which increases the excretion of K+ in this segment further. Thiazides also lead to a long-term decrease in blood pressure. The mechanism for this effect is controversial. A reduction in blood volume would be expected to decrease arterial blood pressure. Blood volume returns to near-normal values, however, after several weeks of thiazide administration. A direct vascular effect—vasodilation caused by reductions in vascular Na+ content—has been proposed to explain the continued reduction in total peripheral resistance that persists during thiazide administration.14
Absorption, fate, and excretion
Absorption of thiazides from the gastrointestinal tract varies with the particular agent. The plasma elimination half-life and duration of diuretic effect for each of the thiazide diuretics are listed in Table 27-2. Plasma protein binding varies considerably among this class of drugs. The parent compounds or metabolites or both are primarily excreted through renal elimination after glomerular filtration and secretion in the proximal tubule.
Therapeutic uses
Thiazide diuretics are primarily used to treat essential hypertension. The Joint National Commission VII and the World Health Organization recommend thiazide diuretics as a first-line treatment for essential hypertension because of their demonstrated efficacy and low cost (see also Chapter 28).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses