Pathology and Management of Periodontal Problems in Patients with Human Immunodeficiency Virus Infection
Acquired immunodeficiency syndrome (AIDS) is characterized by profound impairment of the immune system. The condition was first reported in 1981, and a viral pathogen, the human immunodeficiency virus (HIV), was identified in 1984.221 The condition was originally thought to be restricted to male homosexuals. Subsequently, it was also identified in male and female heterosexuals and bisexuals who participated in unprotected sexual activities or who abused injected drugs.187 Currently, sexual activity and drug abuse remain the primary means of transmission.
HIV has a strong affinity for cells of the immune system, most specifically those that carry the CD4 cell surface receptor molecule. Thus, helper T lymphocytes (T4 cells) are most profoundly affected, but monocytes, macrophages, Langerhans cells, and some neuronal and glial brain cells may also be involved.125 Viral replication occurs continuously in the lymphoreticular tissues of the lymph nodes, the spleen, the gut-associated lymphoid cells, and the macrophages.282,283
In 1986, a second type of HIV was isolated in Western Africa, and subsequently the two were named HIV-1 and HIV-2. Infection with HIV-2 was first reported in the United States in 1987 in an individual who had migrated from Western Africa. The two viruses apparently originated in different simian species. HIV-2 is very similar to HIV-1, but it appears to be less virulent, and it causes AIDS much more slowly. Infection with HIV-2 is rarely identified outside of Africa and countries that have been intimately associated with that area. During subsequent years, various subgroups of the viruses have been identified. HIV-1 consists of three named subgroups: M (main or major), N (new or non-M) and O (outlier). HIV-1 subgroup M is primarily responsible for the worldwide HIV epidemic. There are at least 10 subtypes of HIV-1 M, with subtype B being the most common. It has also been learned that co-infection with varying groups or subgroups can result in circulating recombinant forms of viral strains. There are also eight known HIV-2 subgroups. There is evidence that this growing plethora of viral mutations may play a role in the increasing resistance to antiretroviral therapy. It is known, for example, that HIV-2 possesses a natural resistance to non-nucleoside reverse transcriptase inhibitor antiretroviral drugs, and circulating recombinant forms may also develop resistance to drugs that are being used for treatment.126,163,217
During recent years, combined therapeutic regimens that consist of antiretroviral agents and protease-inhibiting drugs have resulted in marked improvement in the health status of HIV-infected individuals and occasionally a reduction in viral plasma bioloads to below detectable levels (i.e., <50 copies/ml), although the infection may still be transmissible.79,108,114,290 Current evidence indicates that the virus is never completely eradicated; rather, it is sequestered at low levels in resting CD4 cells, even in individuals with no detectable plasma viral ribonucleic acid (RNA).49,78 These findings suggest that effective combination drug therapy may be necessary for the lifetime of infected individuals. Long-term control of the infection may be difficult, because the antiviral agents that are currently used have many adverse side effects and readily develop drug-resistant variant viral strains.282 In addition, growing evidence suggests that oral pathogenic microorganisms (including putative periodontal pathogens) may help to induce HIV recrudescence by reactivating latently infected dendritic cells, macrophages, or T cells.97,123,124
Pathogenesis
In patients with untreated or inadequately treated HIV infection, the overall effect is the gradual impairment of the immune system via interference with T4 (CD4) lymphocytes and other immune cell functions.283 Recent evidence indicates that innate immune response may play a role in controlling HIV replication. Chemokine receptor type 5 (CCR5) is found on the surface of CD4 cells, and it often serves as a point of entry for HIV into the cell. A genetic variation associated with the expression of CCR5 and its ligand has been found to decrease an individual’s susceptibility to HIV infection and to delay clinical progression independent of their effects on viral replication. This may serve to partially explain the lack of progression of HIV infection in some individuals, and it may ultimately lead to a cure for HIV infection.125,253
B lymphocytes are not infected, but the altered function of infected T4 lymphocytes secondarily results in B-cell dysregulation and altered neutrophil function.147 This may place the HIV-positive individual at increased risk for malignancy and disseminated infections with microorganisms such as viruses, mycobacterioses, and mycoses.107,202,210 HIV-positive individuals are also at increased risk for adverse drug reactions as a result of altered antigenic regulation.196
Epithelial cells of the mucosa may become infected, and they may allow for the access of the virus into the bloodstream. Some evidence suggests that oral epithelial cells may harbor HIV virions and infect CD4+ cells by direct cell-to-cell transfer or by transporting and releasing low levels of infectious virions.271 Most evidence, however, indicates that oral transmucosal viral transmission occurs after mild or severe traumatic injury or punctures of the mucous membranes. This allows for the infection of circulating host defense cells such as lymphocytes, macrophages, and dendritic cells.43,244
HIV has been detected in most body fluids, although it is found in high quantities only in blood, semen, and cerebrospinal fluid. Transmission occurs almost exclusively by sexual contact, the illicit use of injection drugs, or exposure to blood or blood products. Transmission after human bites has been reported, although the risk is extremely low.207,277
The high-risk population includes homosexual and bisexual men; users of illegal injection drugs; persons with hemophilia or other coagulation disorders; recipients of blood transfusions before April 1985; infants of HIV-infected mothers (in whom transmission occurs by fetal transmission, at delivery, or by breastfeeding); promiscuous heterosexuals; and individuals who engage in unprotected sex with HIV-positive cohorts.113 Heterosexual transmission is a common cause of AIDS in the world population, and it is increasing significantly in the United States.38,197 Transmission is more likely to occur through contact with HIV-infected individuals harboring a high plasma bioload of the virus.197,223 HIV transmission has also been reported to occur through organ transplantation and artificial insemination.46
Some short-term studies have suggested that HIV-positive individuals who have been successfully treated with antiretroviral therapy (i.e., who have no detectable viral load) cease to be infectious to others.32,208 However, Wilson and colleagues284 have developed a mathematical model that indicates that the potential for HIV transmission to an uninfected individual by a heterosexual partner with an undetectable viral load is low but not without some risk. This risk appears to increase with more frequent exposure and the presence of other sexually transmitted diseases. Incomplete adherence to antiretroviral therapy could further increase the risk of transmission. Unprotected sex in this scenario creates a risk of HIV transmission that is four times greater than that associated with condom use.
The concept of serosorting has been advocated with increasing frequency in an effort to allow unprotected sex among male homosexuals who are both HIV positive or both HIV negative. This is the practice of preferentially having sex only with partners of concordant HIV status while selectively using condoms with HIV-discordant partners. There does appear to be some reduction in risk to others by HIV-positive individuals who only have sex with other seropositive individuals. However, the degree of risk for other sexually transmitted diseases remains high. Among HIV-negative individuals, serosorting presents an increased risk of the transmission of HIV and other sexually transmitted diseases, because some HIV-infected individuals are not aware of their disease status or do not reveal their status. Consequently, the consistent use of condoms is strongly recommended for both HIV concordant and HIV discordant sexual activity.95,122,140
Epidemiology and Demographics
In November 2012, the Foundation for AIDS Research provided worldwide estimates that indicated that more than 34 million people were living with HIV/AIDS, 3.3 million of whom were less than 15 years old. In 2011, an estimated 2.5 million individuals were newly infected, and 1.7 million people had died from AIDS that year. Since the beginning of the epidemic, more than 60 million have contracted HIV, and approximately 30 million have died. Sub-Saharan Africa continues to be the global epicenter of the AIDS pandemic, and 23.5 million individuals in that area (69% of the worldwide total) are living with HIV/AIDS. Ninety-one percent of the world’s HIV-positive children live in the area, and 71% of the worldwide AIDS deaths occurred there in 2011.82 The increase in the numbers of patients living with AIDS in the United States and other developed countries has resulted in part from prolonged survival since the advent of multidrug highly active antiretroviral therapy (HAART).23,79,198,273,276 Thus, AIDS represents the most serious medical crisis in world history.41,82,269
AIDS affects individuals of all ages, but more than 98% of cases occur among adults and adolescents who are more than 12 years old. Centers for Disease Control and Prevention (CDC) estimates indicate that, in 2010, approximately 29,800 newly infected adult victims in the United States were men who had sex with men (MSM), who accounted for 63% of all new infections. Disturbingly, the greatest increase in HIV-infected MSM was seen in the youngest age group (13 to 24 years old), and it was especially prevalent among young black MSM. An additional 4% of the MSM group also used illicit injection drugs. HIV transmission through injection drug use alone infected 12%, whereas 25% of all new HIV infections in the United States were contracted through heterosexual contact, usually with a high-risk individual.35,38 More than 25% of newly infected people were women, the majority of whom had sex with intravenous drug users or bisexual men.39–41,82
Other women with AIDS were born in countries such as Haiti or one of several high-incidence African nations in which heterosexual contact is the major mode of transmission. Only one individual contracted AIDS from blood products or blood transfusions in the United States in 2007 as a result of stringent blood bank controls; this data is no longer maintained as a single entity. This mode of transmission continues to be a threat, however, in undeveloped countries. A disproportionately high number of black/African-American and Hispanic male homosexuals, male and female heterosexuals, and children of affected women have HIV infection. The major risk factor for this disparity appears to be a more frequent history of the use of injection drugs and needle sharing as well as unprotected sexual activity in these groups.35,38,154 It is of interest to note that the mother-to-child transmission of HIV in the United States has progressively decreased since its peak in 1992 because of the use of antiretroviral therapy.264
Transmission from health care workers to patients has been documented on three occasions, with one dentist infecting six patients either accidentally or deliberately.146,161 Conversely, seroconversion has been documented in 103 health care workers after occupational injury, usually related to the management of patients with high plasma viral loads. The majority of these incidents involved nurses, and no documented seroconversion has been reported among dental health care workers.81,128,161,289
Classification and Staging
Clinical stage 1: Asymptomatic infection or persistent generalized lymphadenopathy
Clinical stage 2: Mild symptoms such as mild unexplained weight loss, angular cheilitis, herpes zoster, recurrent oral ulcerations, papular pruritic eruptions, seborrheic dermatitis, fungal infections, and recurrent respiratory infections
Clinical stage 3: Advanced symptoms such as severe weight loss, chronic diarrhea, persistent intermittent or constant fever, pulmonary tuberculosis, severe bacterial infections, unexplained anemia, neutropenia, chronic thrombocytopenia persistent oral candidiasis, oral hairy leukoplakia, acute necrotizing stomatitis, gingivitis, and periodontitis
Clinical stage 4: Severe symptoms such as HIV wasting syndrome, Pneumocystis pneumonia, chronic herpes simplex infection of more than 1 month’s duration, esophageal candidiasis, extrapulmonary tuberculosis, Kaposi’s sarcoma, cytomegalovirus (CMV) infection, lymphoma, candidiasis (of the trachea, bronchi, or lungs), invasive cervical carcinoma, and others281
AIDS may be present with either clinical stage 3 or stage 4 disease. A CD4 count of less than 350/mm3 is considered diagnostic for AIDS in adults and in children who are 5 years old or older. Advanced HIV infection in infants is based on a CD4+ cell value of less than 30% if the patient is less than 12 months old; of less than 25% among those between 12 and 35 months old; and of less than 20% among those between the ages of 36 and 59 months. (These are the WHO case definitions of HIV for surveillance and the revised clinical staging and immunologic classifications of HIV-related disease in adults and children.3,36)
In 1982, the CDC developed a surveillance case definition for AIDS based on the presence of opportunistic illnesses or malignancies secondary to defective cell-mediated immunity in HIV-positive individuals.36,37 The 1993 revision added invasive cervical cancer in women, bacillary tuberculosis, and recurrent pneumonia to the AIDS designation. Currently, any of 25 specific clinical conditions found in HIV-positive individuals can establish the diagnosis of AIDS (Box 26-1). The most significant change in the most recent CDC case definition was the inclusion of severe immunodeficiency (CD4+ T lymphocyte count of <200/mm3 or CD+ T lymphocyte percentage of <14% of total lymphocytes) as being definitive for AIDS. This change was based on the recognition that severe immunodeficiency results in an increased risk for opportunistic, life-threatening conditions. Many HIV-positive individuals have been designated as patients with AIDS solely because of a low CD4 cell count or percentage, even in the absence of life-threatening conditions. More recently, HIV plasma bioload has been identified as a significant factor related to the transmissibility and severity of the disease.
HIV infection among children less than 13 years old is based on immunologic categories. Category N infected children show no evidence of suppression; category A designates evidence of mild suppression; category B designates moderate suppression; and category C represents severe symptomatic suppression. Perinatally exposed infants are usually not categorized until their HIV status is determined at the age of 18 months, because standard anti-HIV immunoglobulin G antibody tests are not reliable for patients younger than this age. Infants born to HIV-positive mothers usually possess maternal anti-HIV immunoglobulin G antibodies, which cross the placental barrier and offer some perinatal protection. Despite this, 15% to 30% of these infants ultimately become infected with HIV.41
Testing for Human Immunodeficiency Virus Infection
The enzyme-linked immunosorbent assay (ELISA) and Western blot (WB) tests were the original methods for determining the presence of HIV-1 and HIV-2 antibodies in serum or plasma, and they are still considered the gold standard of confirmatory testing. The ELISA test is performed first, and it is repeated if the first test is positive. If it is positive a second time, WB is performed, and a positive finding is considered diagnostic for the infection. The WB is based on the reactivity of antibodies to specific viral proteins. It tests for the proteins p24 (capsid protein), gp41, and gp120 through gp160. In many laboratories, a positive band to two of these three proteins is considered positive, whereas anything less is considered indeterminate or negative if no antigenic proteins are detected. Both ELISA and WB require the presence of circulating HIV antibodies, which may take several weeks or even months to become detectible. Thus, false-negative tests are possible and repeat testing may be required, although modern testing methods are highly accurate. Today, several other test methods are based on the presence of an HIV antigen. Although these tests are expensive, they may be of benefit, especially for early diagnosis after exposure.42,65,75
The window of time between exposure and the development of antibodies can lead to an increased risk for disease transmission, and some evidence suggests that early treatment during that window can significantly minimize the intensity of the infection and positively influence treatment outcomes. Consequently, during recent years, several test methods have been developed that detect the presence of HIV genes, RNA, or deoxyribonucleic acid (DNA). These tests may offer the advantage of the earlier confirmation of recent exposure and infection. Several rapid HIV tests are currently available that can provide results in minutes as opposed to days. In some instances, collection kits are available for personal use, including one that collects oral fluids. At this time, the US Food and Drug Administration has approved approximately seven of these rapid tests as well as three that test for HIV and for coinfection with the hepatitis C virus; one of these also tests for hepatitis B virus. Rapid tests may require the collection of oral fluids, plasma, serum, or whole blood, and the CDC encourages their use in most health care settings. It must be recognized that rapid tests may have a relatively high number of false-negative results as well as some false-positive results, so repeat testing and follow-up testing with ELISA and WB are often indicated.75 Rapid tests may be of special benefit in undeveloped countries, where medical laboratory testing may not be readily available.42,58,65
The number of individuals living with AIDS in the United States has greatly increased during recent years as a result of the development of HAART, which combines various types of antiretroviral drugs, protease inhibitors, and fusion inhibitors.110,132 Infected individuals treated with HAART may experience a marked rise in CD4 cell levels and a decreased plasma viral load. CD4 counts may reach normal levels, and viral bioload may decrease to a point below the level of detection.19,201 Despite this improvement, these individuals are still considered to have AIDS, because the virus is apparently sequestered somewhere in the body. The patient remains potentially infectious to others, and viral activity may resume if medications are discontinued or if severe coinfections with sexually transmitted diseases or other diseases occur.78,83,127,221,272,273 Some evidence suggests that severe oral infections, including periodontal diseases, may sometimes represent a significant coinfection.
A few weeks to a few months after initial exposure, some HIV-infected individuals may experience acute symptoms, such as the sudden onset of an acute mononucleosis-like illness that is characterized by malaise, fatigue, fever, myalgia, erythematous cutaneous eruption, oral candidiasis, oral ulcerations, and thrombocytopenia.83,160,272 This acute phase may last for up to 2 weeks, with seroconversion occurring 3 to 8 weeks later.273 However, antigenic viremia may sometimes be present for an extended time before seroconversion occurs.127,145 Some individuals experience asymptomatic HIV infection, whereas others may become asymptomatic after the initial acute infection. In either case, infected individuals eventually become seropositive for HIV antibody, but the mean time from infection until the development of AIDS is now estimated to be up to 15 years or more.127,145
Centers for Disease Control and Prevention Surveillance Case Classification
Patients with AIDS have been grouped as follows, according to the 1993 CDC Surveillance Case Classification36:
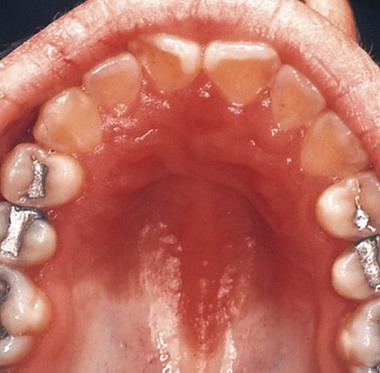
• Category A includes patients with acute symptoms or asymptomatic diseases as well as individuals with persistent generalized lymphadenopathy with or without malaise, fatigue, or low-grade fever (Figure 26-1).
• Category B patients have symptomatic conditions such as oropharyngeal or vulvovaginal candidiasis, herpes zoster, oral hairy leukoplakia, and idiopathic thrombocytopenia or constitutional symptoms of fever, diarrhea, and weight loss.
• Category C patients are those with outright AIDS as manifested by life-threatening conditions or as identified by CD4+ T lymphocyte levels of less than 200 cells/mm3.
The CDC staging categories reflect progressive immunologic dysfunction, but patients do not necessarily progress serially through the three stages, and the predictive value of these categories is not known. However, because HAART drugs may cause severe adverse side effects, many AIDS treatment centers continue to follow these guidelines to determine when to initiate or discontinue multidrug therapy.15,27,30,111
Antiretroviral Therapy
To date, 36 drugs or drug combinations from six separate drug classes have been approved by the US Food and Drug Administration for the treatment of HIV and AIDS infection (Box 26-2). However, two of these drugs, Hivid and Fortovase, are no longer marketed.1 The goals of therapy are to reduce morbidity and mortality and to improve and prolong life. To achieve this, it is necessary to suppress plasma viremia and to improve or preserve immunologic function. However, the total eradication of HIV does not appear to currently be possible, because reservoirs of latently infected CD4 cells are established early during the infection and persist even with treatment.185 Anatomic reservoirs of infection have been located in the gastrointestinal and reproductive tracts and in breast, lung, and brain tissue. Recently, the oral cavity has been proposed as an anatomic reservoir, because a small percentage of HIV-infected individuals were found to be salivary hyperexcretors of HIV.244
Protease inhibitors are active against both HIV-1 and HIV-2. They bind to the HIV protease enzyme and result in the formation of noninfectious HIV virions. Fusion inhibitors bind to the gp41 glycoprotein membrane on HIV and prevent virus fusion into susceptible CD4 cells. The drugs are administered by subcutaneous injection.83,188,268 Some entry inhibitors block the access of HIV to CCR5 receptors on macrophages and to T cells that are helping to preserve immune function and reduce viral load.2 Integrase inhibitors block the incorporation of viral DNA into CD4 cell DNA after viral RNA has been converted into DNA by the reverse transcription enzyme.252 Combination antiviral agents are composed of multiple drugs with various sites of viral disruption of incorporation into CD4 cells. These combinations represent the transformation of antiretroviral therapy into HAART.188
NNRTIs are potent, but they may lead to the rapid development of resistance and cross-resistance to all drugs in the category. The NNRTIs have the potential for adverse interactions with other HIV drugs, especially protease inhibitors, and adverse drug interactions are common with all drugs that are metabolized by the hepatic p450 system, principally the CYP3A4 enzyme. Stevens–Johnson syndrome may develop, and hepatic and central nervous system manifestations are common.196
Protease inhibitors are potent antiretrovirals that also are metabolized by the CYP3A4 isoenzyme of the hepatic p450 system, which means that adverse drug interactions are common to other antiretroviral agents and non-HIV agents. Gastrointestinal intolerance, hyperlipidemia, insulin resistance, and lipodystrophy are common.188,268
The only fusion inhibitor that has currently been approved is enfuvirtide. Because it is administered subcutaneously, injection site reactions are common. Enfuvirtide is rarely used alone for the management of HIV infection.283
Highly Active Antiretroviral Therapy
With HAART therapy, combinations of three or more antiretroviral drugs are administered simultaneously for long periods of time in an effort to block HIV replication and plasma viral load and to restore immune function while minimizing antiretroviral drug resistance and adverse drug effects. The goals are the improvement of the patient’s quality of life and the reduction of HIV-related morbidity and mortality. Three commonly used combinations are one NNRTI plus two NRTIs; one or two protease inhibitors plus two NRTIs; or three NRTIs. One NRTI three-drug combination is available in a single tablet, but this antiretroviral therapy does not necessarily qualify as HAART.80,196
It is now nearly 20 years since the advent of HAART, and some authorities propose that drug combinations be personalized for each HIV-positive individual on the basis of the specific characteristics of the drugs (e.g., toxicity, potency, central nervous system/sanctuary site penetration); the virus (e.g., resistance pattern, history of antiretroviral use, CCR5 tropism); and the host (e.g., lifestyle, compliance, drug interactions, non-AIDS comorbidities, organ dysfunction, pregnancy).196
Considerable controversy has existed for several years with regard to determining the best time to initiate HAART. Some authorities felt that the drugs should not be administered until significant immune suppression occurred. It was thought that this approach would minimize the side effects as well as the risk of developing drug resistance. Others advocated the initiation of therapy as soon as the diagnosis was established. More recently, evidence has indicated that early treatment is far more successful than delayed treatment.20,196
HAART has to be proven very successful for achieving a significant increase in the survival and quality of life of HIV-infected individuals, and life expectancy is markedly increased for many individuals using this therapy.4 However, not everyone benefits equally from HAART. Survival time is shorter despite HAART among individuals who are injected drug abusers, in those with AIDS before the initiation of therapy, in those with a very high viral load before the initiation of therapy (i.e., >100,000 copies/ml), in those who are inconsistent in their adherence to the drug regimen, and in those who discontinue the drugs. Other factors that affect life expectancy in the era of HAART include smoking; excessive alcohol consumption; older age at initiation of therapy; comorbidities such as hepatitis C or other viral, bacterial, or fungal infections; chronic liver, kidney, or cardiovascular conditions; and diabetes mellitus. Low socioeconomic status is also a negative factor that is perhaps related to access to care, and HIV-related death continues to occur earlier among Hispanics and non-Hispanic blacks.69,97
Before the advent of antiretroviral therapy, opportunistic infections were the principal cause of morbidity and mortality among HIV-infected persons. Even today, opportunistic infections continue to occur among those who are unaware of their HIV infection, those who do not take recommended medications, and those who are resistant or who react adversely to recommended medications. In some instances, HAART has not altered the rate of occurrence or severity of certain opportunistic infections, whereas it is associated with the increased occurrence of others. Commonly described systemic conditions that may be found more often after HAART are lipodystrophy, increased insulin resistance, gynecomastia, blood dyscrasias, and dermatologic disorders. In the oral cavity, candidal infections are markedly reduced among individuals who are responsive to antiretroviral therapy261; hairy leukoplakia, Kaposi’s sarcoma, deep mycoses, necrotizing ulcerative gingivitis (NUG), and necrotizing ulcerative periodontitis (NUP) are reduced in these patients as well. Conversely, the incidence and severity of oral condylomata and HSV infection appear unchanged, whereas neutropenia-associated oral aphthous-like ulcers; lichenoid reactions; and oral melanotic macules of the lip, gingiva, palate, or buccal mucosa may increase.238 On the basis of these observations, the presence or absence of oral opportunistic infections associated with HIV infection or its treatment may serve as markers for the success or failure of antiretroviral therapy.6,9,18,56,67,102,107,175,219,233,255,259
Immune Reconstitution Inflammatory Syndrome
Complications from rapid immune recovery have been described for many years, usually among individuals with bone marrow transplants and cancer chemotherapy. However, this phenomenon has been identified as a common complication associated with the recovery of immunity that occurs with HAART, and it has been termed the immune reconstitution inflammatory syndrome (IRIS). In patients with this syndrome, pre-existing subclinical or minimally symptomatic infections worsen. Recurrent lesions of Kaposi’s sarcoma may be particularly ominous. More often, infectious diseases (e.g., tuberculosis, disseminated Mycobacterium avium complex disease, hepatitis B, hepatitis C, herpes zoster viruses) are reactivated, which causes an overwhelming inflammatory response that makes the symptoms of infection more severe. The triggering antigen may be a viable infectious agent (i.e., unmasking form) or even a dead or dying infectious organism; a tumor antigen; or a host autoimmune antigen (i.e., paradoxical form).266 In most instances, a single antigen is responsible for the condition. However, it occasionally presents with multiple manifestations.2 IRIS seems to occur among individuals with low baseline CD4 counts who are responsive to HAART, and it causes a rapid increase in the CD4 cell numbers and a hyperinflammatory response that may cause fever and worsening of an existing bacterial, viral, or fungal infection.248 In the oral cavity, IRIS lesions could include CMV infection, varicella-zoster virus infection, histoplasmosis, cryptococcal infections, Kaposi’s sarcoma, and other conditions. Although sometimes described years after the initiation of ART, IRIS most often occurs within the first 2 to 12 weeks after starting ART in individuals with rapidly decreasing HIV viremia and rapidly increasing CD4+ T lymphocyte counts; in individuals who are starting ART for the first time; in those who are changing their treatment regime; or in those who were not compliant and discontinued their ART treatment for a period of time and then restarted therapy. The condition is difficult to diagnosis and manage, and it may take months to subside, but it does not appear to affect patient survival in most instances.l However, as mentioned previously, recurrent Kaposi’s sarcoma may indicate a grim prognosis. At this time, the implications of IRIS as the apply to oral opportunistic infections are largely unknown, but parotid enlargement has been reported as a common oral manifestation for reasons that have not yet been elucidated.120,184,185,216
Oral and Periodontal Manifestations of Human Immunodeficiency Virus Infection
Oral lesions are common in HIV-infected patients, although geographic and environmental variables may exist. Previous reports have indicated that most patients with AIDS have head and neck lesions,223 whereas oral lesions are quite common among HIV-positive individuals who do not yet have AIDS.16,93 Several reports have identified a strong correlation between HIV infection and oral candidiasis, oral hairy leukoplakia, atypical periodontal diseases, oral Kaposi’s sarcoma, and oral non-Hodgkin’s lymphoma.68,71,153
Oral lesions less strongly associated with HIV infection include melanotic hyperpigmentation, mycobacterial infections, necrotizing ulcerative stomatitis (NUS), miscellaneous oral ulcerations, and viral infections (e.g., herpes simplex virus [HSV], herpes zoster, and condyloma acuminatum). Lesions that are seen in HIV-infected individuals with undetermined frequency include less common viral infections (e.g., CMV, molluscum contagiosum), recurrent aphthous stomatitis, and bacillary angiomatosis (epithelioid angiomatosis).68
The advent of HAART has resulted in the greatly diminished frequency of oral lesions associated with HIV infection and AIDS.34,179,261
However, some medical protocols delay the use of HAART drugs until immune suppression is relatively severe. These protocols also discontinue HAART if immune stability has been achieved or if the targeted virus becomes drug resistant.74,167,233,262 In theory, these protocols suggest that patients with HIV and AIDS may experience a greater number of oral manifestations than patients who start HAART therapy earlier. The dental practitioner must continue to be prepared to diagnose and manage these conditions in coordination with the patient’s physician. However, HIV resistance to antiretroviral drug therapy is an ongoing problem, and this resistance may even be transmitted to newly infected sexual partners or to newborns of infected mothers.87
Oral Candidiasis
Candida, a fungus that is found in the normal oral flora, proliferates on the surface of the oral mucosa under certain conditions. A major factor associated with the overgrowth of Candida is diminished host resistance, which is seen in debilitated patients or in patients who have received immunosuppressive therapy. The incidence of candidal infection has been demonstrated to increase progressively in relationship to diminishing immune competency.158,159,249 Most oral candidal infections (i.e., 85% to 95%) are associated with Candida albicans, but other species of Candida may also be involved. Currently, at least 11 strains of Candida have been identified, and non–Candida albicans infections are more common among immunocompromised individuals who are already receiving antifungal therapy for C. albicans.155,236
Candidiasis is the most common oral lesion associated with HIV disease, and it has been found in approximately 90% of patients with AIDS.194,246 It usually has one of four clinical presentations: pseudomembranous, erythematous, or hyperplastic candidiasis or angular cheilitis.115,178
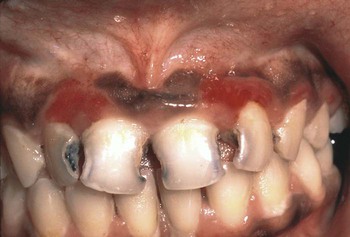
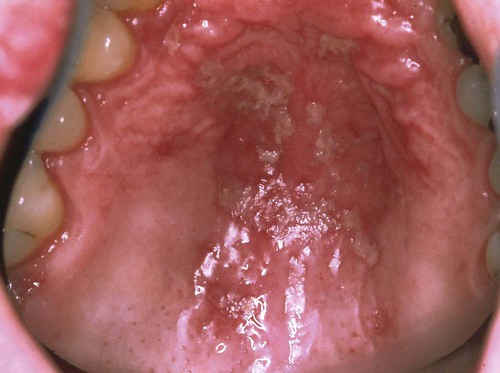
Pseudomembranous candidiasis (“thrush”) presents as painless or slightly sensitive, yellow-white, curdlike lesions that can be readily scraped and separated from the surface of the oral mucosa. This type is most common on the hard and soft palate and the buccal or labial mucosa, but it can occur anywhere in the oral cavity (Figure 26-2).
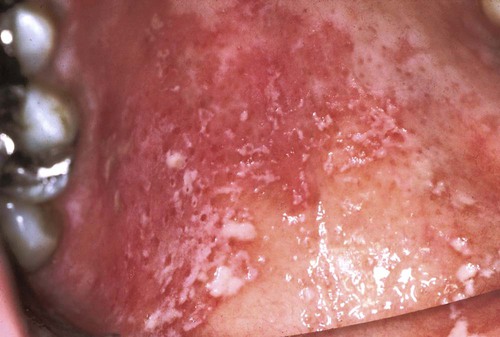
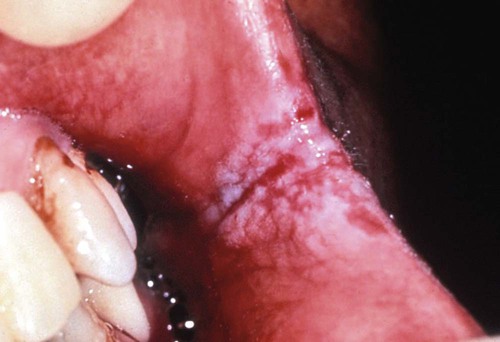
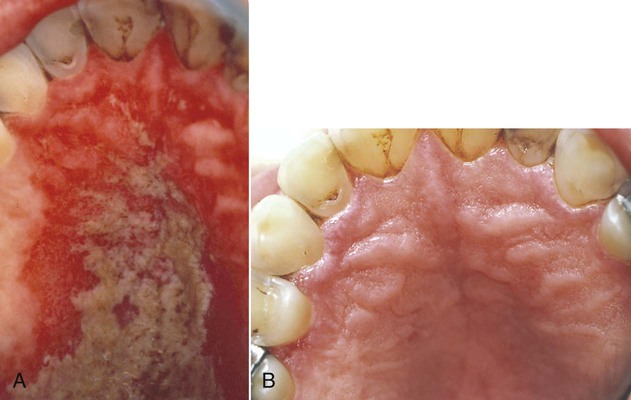
Erythematous candidiasis may be present as a component of the pseudomembranous type, appearing as red patches on the buccal or palatal mucosa, or it may be associated with depapillation of the tongue. If the gingiva is affected, it may be misdiagnosed as desquamative gingivitis (Figures 26-3, 26-4, and 26-5). Some evidence suggests that erythematous candidiasis in more common among HIV-positive individuals with CD4+ counts between 200 and 500 cells/µl, whereas the pseudomembranous form occurs in patient with less than 200 CD4+ cells/µl. Viral load is most often elevated to more than 100,000 copies/ml. However, it should be emphasized that any form of candidiasis can be found in individuals who are only minimally immunocompromised.181
Hyperplastic candidiasis is the least common form, and it may be seen in the buccal mucosa and the tongue. It is more resistant to removal than the other types (Figures 26-6 and 26-7).
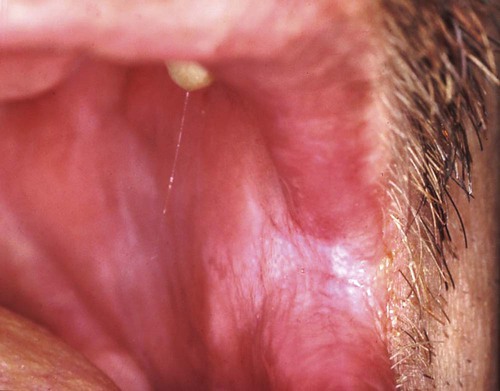
The diagnosis of candidiasis is made by the clinical evaluation, culture analysis, or microscopic examination of a tissue sample or smear of material scraped from the lesion (oral cytology), which shows hyphae and yeast forms of the organisms (Figure 26-8). When oral candidiasis appears in patients with no apparent predisposing causes, the clinician should be alerted to the possibility of HIV infection.68 Many patients who are at risk for HIV infection who present with oral candidiasis also have esophageal candidiasis, which is a diagnostic sign of AIDS.263
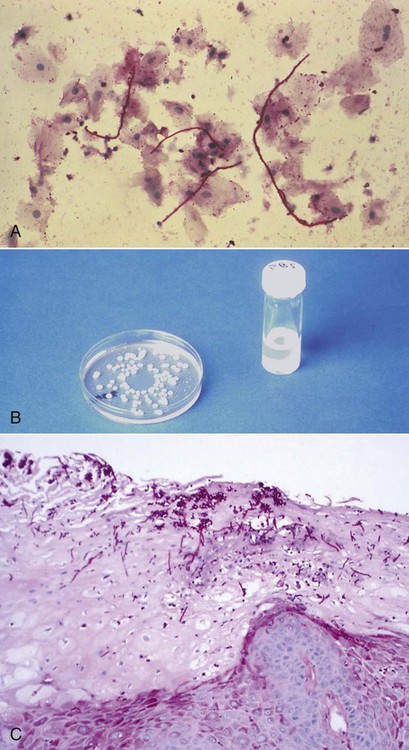
Although oropharyngeal candidiasis occurs with some frequency among immunocompetent individuals, it and esophageal candidiasis are especially common among HIV-infected people. Some evidence suggests that the Th17 subset of T helper cells may be very important to the resistance of infections caused by common oral commensal microorganisms. Its selective loss in HIV infection may allow candidal species to express their pathogenic potential by creating an environment that allows for the overexpression of fungal virulence factors that can degrade host defense components, such as histatin-5 and E-cadherin. Some evidence suggests that the fungus may actually bind to the HIV proteins.31 Because non–C. albicans candidal species appear to be especially prone to the development of azole resistance, species identification may become more important for the control of candidiasis.136 A study from India reported that oral candidiasis infections are more common among males as compared with females, although 92% of infected men and 95% of infected women reported acquiring the infection through heterosexual contact.215 Others have shown that previous ART reduced the risk for oral candidiasis, whereas smoking and the presence of removable full or partial dentures increased the risk.285 Although the incidence of oral candidiasis has been reported to be significantly decreased after HAART treatment, colonization and oral infection remain common among HIV-positive individuals of all ages, either as evidence of unsuccessful therapy or as a result of IRIS.180,192,205,260
Although candidiasis in HIV-infected patients may respond to antifungal therapy, it is often refractory or recurrent. In the past, 30% of patients with AIDS-related candidiasis relapsed within 4 weeks of treatment, and 60% to 80% relapsed within 3 months. This relapse may have resulted from decreasing immunocompetency or the development of antifungal-resistant candidal strains.117 As many as 10% of candidal organisms become resistant to long-term fluconazole therapy, and cross-resistance to other azoles (i.e., ketoconazole, itraconazole, miconazole, or clotrimazole) and amphotericin B may develop.51,135,176,184,186,198,200,210,230,278 Resistant candidiasis is more common among late-presenting individuals with AIDS who have low CD4 counts and high viral loads at baseline.117 Non–C. albicans candidal species in HIV-positive individuals most often include Candida glabrata, Candida tropicalis, Candida krusei, Candida parapsilosis, Candida lusitaniae, Candida guilliermondii, and other as yet unidentified strains. Individuals with recurrent episodes of oropharyngeal candidiasis and previous exposure to antifungal drugs were more likely to colonize non–C. albicans species and to develop azole resistance.170
Recent reports indicate that the administration of HAART to patients with HIV infection has resulted in a significant decrease in the incidence of oropharyngeal candidiasis and oral candidal carriage, and it has also reduced the rate of fluconazole resistance.33,55,67,155,176,179,220 When esophageal candidiasis is present during HAART, there is an increased incidence of non–C. albicans species and fluconazole-resistant organisms.263 Early oral lesions of HIV-related candidiasis are usually responsive to topical antifungal therapy (Figure 26-9). More advanced lesions, including hyperplastic candidiasis, may require systemic antifungal drugs; systemic therapy is mandatory for esophageal candidiasis235,242 (Figure 26-10).
With any therapy, lesions tend to recur after the drug is discontinued, and resistant strains of candidal organisms have been described, especially with the use of systemic agents.62,235,274 Box 26-3 identifies the therapeutic agents that are often prescribed for the treatment of candidal infections. Most oral topical antifungal agents contain large quantities of sucrose, which may be cariogenic after long-term use. For this reason, some authorities recommend the oral use of vaginal tablets because they do not contain sucrose. However, such tablets are relatively low in active units (100,000) as compared with usual oral dosages of 200,000 to 600,000 units. Sucrose-free nystatin is also available in a powder form, which may be mixed extemporaneously with water at each use (i.e., 1 teaspoon of powder in a glass of water). In addition, many compounding pharmacies can provide sucrose-free nystatin on request. Recently, sucrose-free oral suspensions of itraconazole and amphotericin B oral rinse have become available. To date, few comparative studies have been performed to address the effectiveness of these products. Amphotericin B oral suspension is more effective against C. albicans than it is against other species. Patients should be instructed to rinse with the oral suspension for several minutes and then swallow it.206 Fluconazole oral suspension has been reported to be more effective as an antifungal than liquid nystatin. Chlorhexidine and cetylpyridinium chloride oral rinses may also be of some prophylactic value against oral candidal infection.88 The long-term prophylactic effectiveness of once-weekly systemic fluconazole also has been described.235
Systemic antifungal agents such as ketoconazole, fluconazole, itraconazole, and amphotericin B are effective for the treatment of oral candidiasis186 (see Box 26-3). Fluconazole may be the agent of choice when systemic therapy is required.7,61,241,246 As previously mentioned, however, resistant strains of candidal organisms may develop with the prolonged use of any systemic agent, potentially rendering the drugs ineffective against life-threatening candidal infections during the later stages of immunosuppression.29,211 In addition, significant adverse side effects may occur with the long-term use of any systemic antifungal agent. For example, the long-term use of ketoconazole may induce liver damage in individuals with pre-existing liver disease. The increased risk of chronic hepatitis B or hepatitis C infection in immunosuppressed individuals may put some patients at risk for ketoconazole-induced liver damage. If ketoconazole is prescribed, patients should receive liver function tests at baseline and at least monthly during therapy. The drug is contraindicated if the patient’s aspartate transaminase level is more than 2.5 times higher than normal.270 Ketoconazole absorption may also be impaired by the gastropathy experienced by many HIV-infected individuals.141
Oral Hairy Leukoplakia
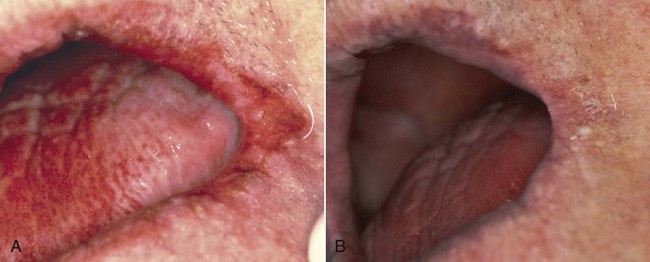
Oral hairy leukoplakia (OHL) primarily occurs in persons with HIV infection.101,104 It is found on the lateral borders of the tongue, it frequently has a bilateral distribution, and it may extend to the ventrum. OHL is caused by the Epstein–Barr virus (EBV), and it is the only EBV lesion with which viral shedding in saliva is common.181 The lesion is characterized by an asymptomatic, poorly demarcated, keratotic area that ranges in size from a few millimeters to several centimeters (Figure 26-11, A). Often, characteristic vertical striations are present, and these impart a corrugated appearance; the surface may also be shaggy and appear “hairy” when dried. The lesion does not rub off, and it may resemble other keratotic oral lesions.
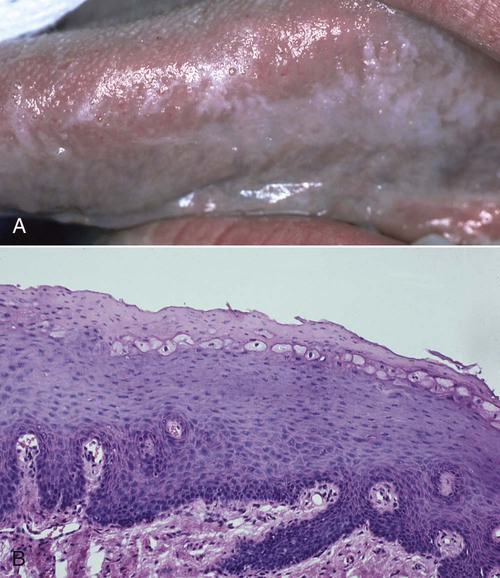
Microscopically, the OHL lesion shows a hyperparakeratotic surface with projections that often resemble hairs. Beneath the parakeratotic surface, acanthosis and some characteristic “balloon cells” that resemble koilocytes are seen (Figure 26-11, B). It has been demonstrated that these cells contain viral particles of the human herpesvirus group; these particles have been interpreted as EBV.101,105
Epithelial dysplasia is not a feature, and, in most OHL lesions, little or no inflammatory infiltrate in the underlying connective tissue is present.232
OHL is found almost exclusively on the lateral borders of the tongue, although it has been reported on the dorsum of the tongue, the buccal mucosa, the floor of the mouth, the retromolar area, and the soft palate.72,101,135 In addition, most of these lesions reveal surface colonization by Candida organisms, which are secondary invaders and not the cause of the lesion.
OHL was originally thought to be caused by the human papillomavirus, but subsequent evidence has indicated an association with EBV.72,101,103,279 During the late 1980s, so-called pseudo–hairy leukoplakia was described in HIV-negative and EBV-negative individuals with lesions that are clinically identical to OHL. In addition, several case reports have described OHL in EBV-infected but HIV-negative individuals with a variety of immunosuppressed conditions (e.g., acute myelogenous leukemia) or who develop immunosuppression as a result of organ transplantation or extensive systemic corticosteroid therapy.103,257 Regardless of the cause, biopsy identification of a lesion that is suggestive of OHL should dictate that HIV testing be performed. It should be emphasized that the severity of the lesion is not correlated with the likelihood of developing AIDS; thus, small lesions are as diagnostically significant as extensive lesions.190
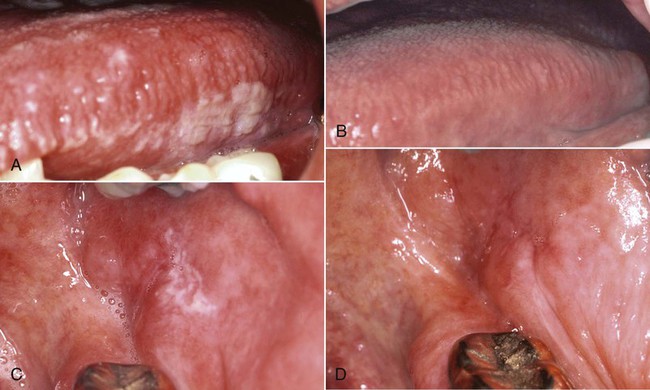
The differential diagnosis of OHL includes several white lesions of the mucosa, such as dysplasia, carcinoma, frictional and idiopathic keratosis, lichen planus, tobacco-related leukoplakia, secondary syphilis, psoriasiform lesions (e.g., geographic tongue), and hyperplastic candidiasis.5,191 The microscopic confirmation of OHL of the tongue in a high-risk patient is considered to be a specific early sign of HIV infection and an indication that the patient will develop AIDS.104,225 Before the advent of effective therapy for HIV, survival analysis indicated that 83% of HIV-infected patients with hairy leukoplakia would develop AIDS within 31 months, and the number of patients with hairy leukoplakia who eventually developed AIDS approached 100%.104 The use of HAART, however, has resulted in a greatly diminished incidence of OHL18,34,74,262,280 (Figure 26-12).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses