Children with Chronic Health Conditions: Implications for Oral Health
Göran Dahllöf, Pernille Endrup Jacobsen, and Luc Martens
Over the past few decades chronic health conditions (CHCs) and disabilities among children and youth have steadily risen primarily for common conditions such as asthma, obesity, mental health conditions, and neurodevelopmental disorders. With new technology, better drugs, and more efficient use of existing treatments an increasing number of children survive their CHCs. In industrialized countries, over 85% of children with CHCs will survive at least to 20 years of age. Other important changes are that children with CHCs are not institutionalized as frequently as in earlier times, they receive early stimulation, and they are usually integrated in the schooling system and attend outpatient medical and dental clinics. Yet, many of these children have not been cured or they will have disabling sequelae of their disease or treatment. Managed care protocols including oral care are at present being developed for several CHCs. For example, in programs for children treated with stem cell transplantation it is recommended that they should be referred to the dentist prior to the start of cytotoxic therapy for oral health information as well as evaluation of the oral health status, particularly the presence of infectious foci. In some instances, oral diseases and also dental care may be life‐threatening to the child, e.g., surgical treatment in children with blood or bleeding disorders. A close collaboration with the medical treatment team is an integral part of the management of oral health care in children with CHC.
General dentists will increasingly meet children with CHCs in their practices and up‐to‐date knowledge of how a CHC affects the oral health of a child is an important aspect of pediatric dental care.
Definition of chronic health conditions
A modern definition of CHC in children focuses on the consequences of the disorder and is independent of diagnosis [1]. Three elements must coexist for a child to be classified as having a CHC (Box 23.1). The duration of a condition can be difficult to predict, particularly when the onset is recent. Disease pattern is defined as the relative consistency or permanence of symptoms or consequences with time. Five patterns of disease have been identified (see Box 23.2 and Figures 23.1 to 23.5).
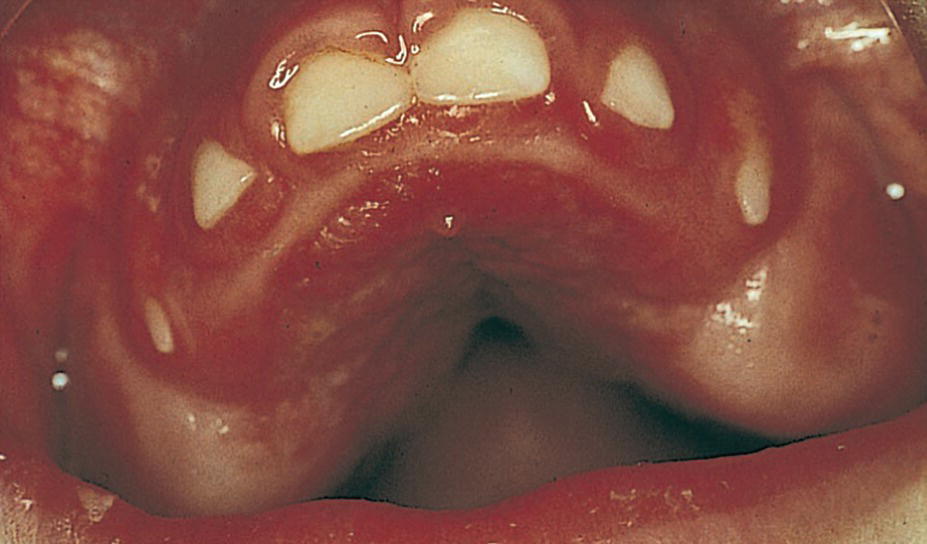
Figure 23.1 A 3‐year‐old boy with cerebral palsy and showing severe gingival overgrowth.
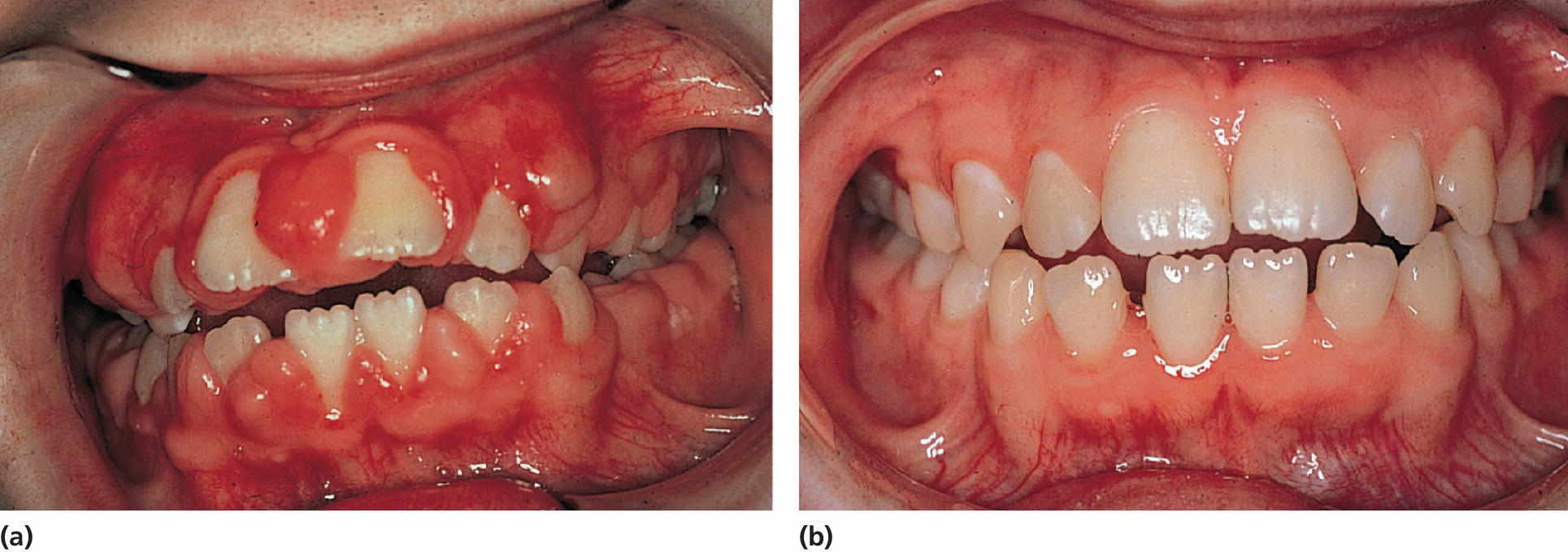
Figure 23.2 (a) A 10‐year‐old girl on phenytoin medication exhibiting severe gingival overgrowth. (b) At 13 years of age, exhibiting a normal gingival 1 year after gingivectomy and discontinuation of phenytoin medication.
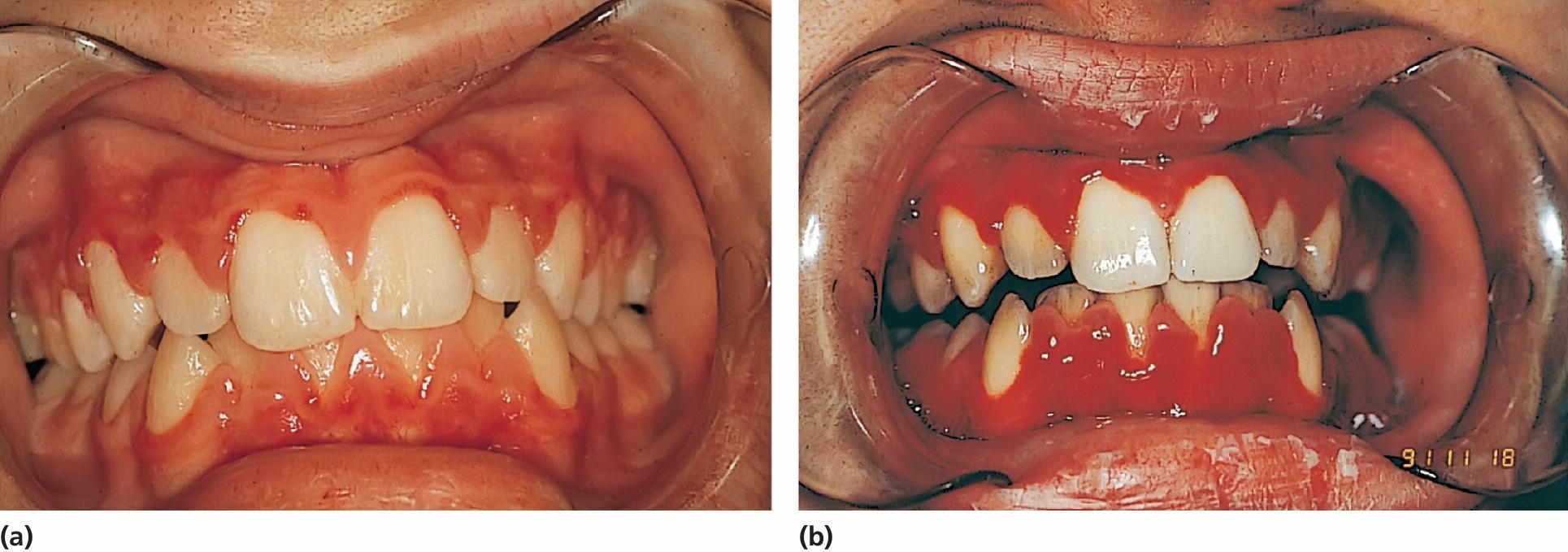
Figure 23.3 (a) A 15‐year‐old boy with aplastic anemia prescribed cyclosporine medication. (b) At 18 years of age, deteriorating gingival health coincidental with a hematological crisis.
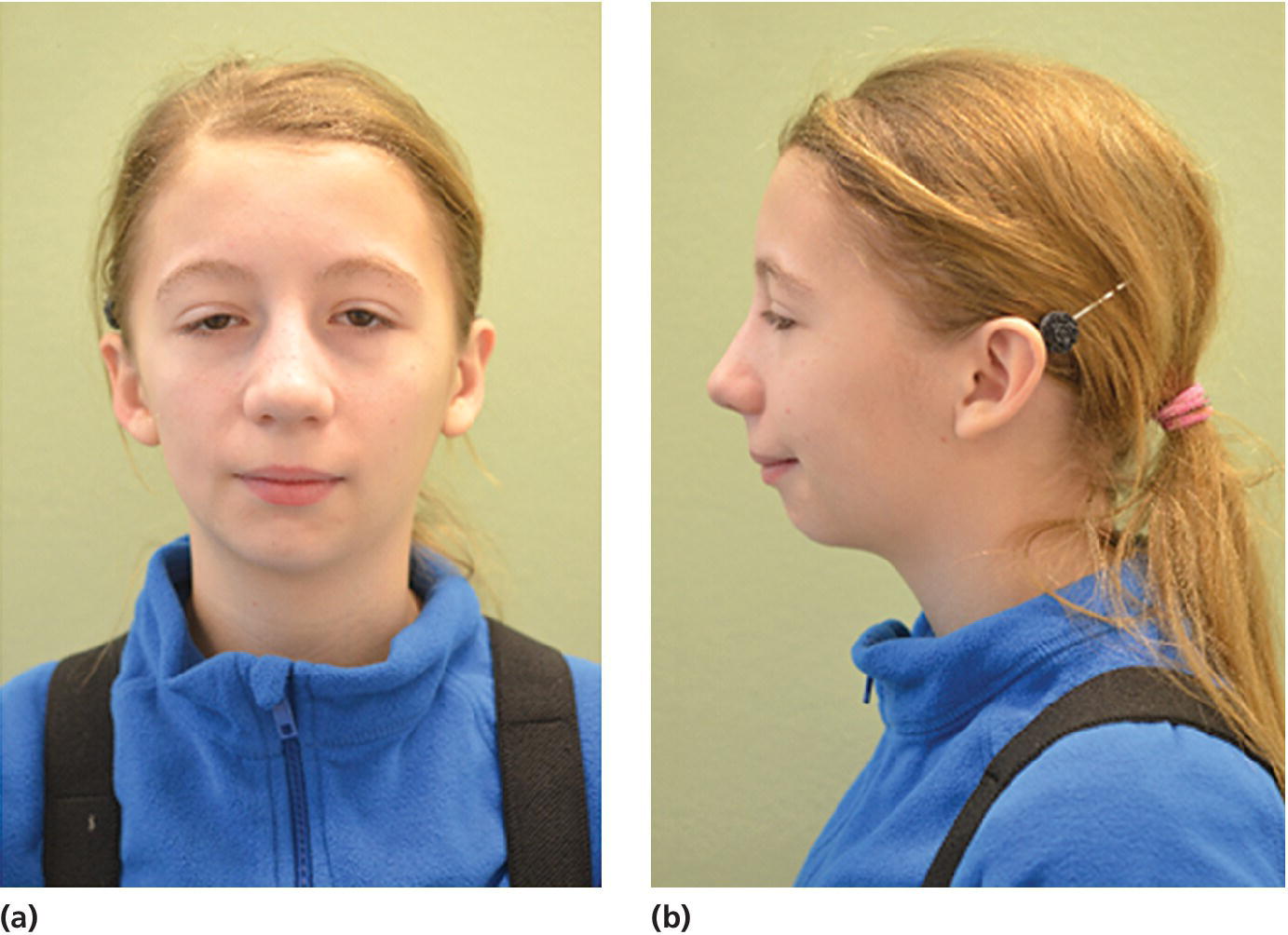
Figure 23.4 A 14‐year‐old girl with restricted and asymmetric growth of the mandible due to unilateral TMJ arthritis in her right side.
(Courtesy of Professor Thomas Klit Pedersen, and Specialist in Orthodontics Anne Agger Mortensen, Division for Dento‐ and Craniofacial anomalies, Section of Orthodontics, Department of Dentistry, Health, University of Aarhus, Denmark.)
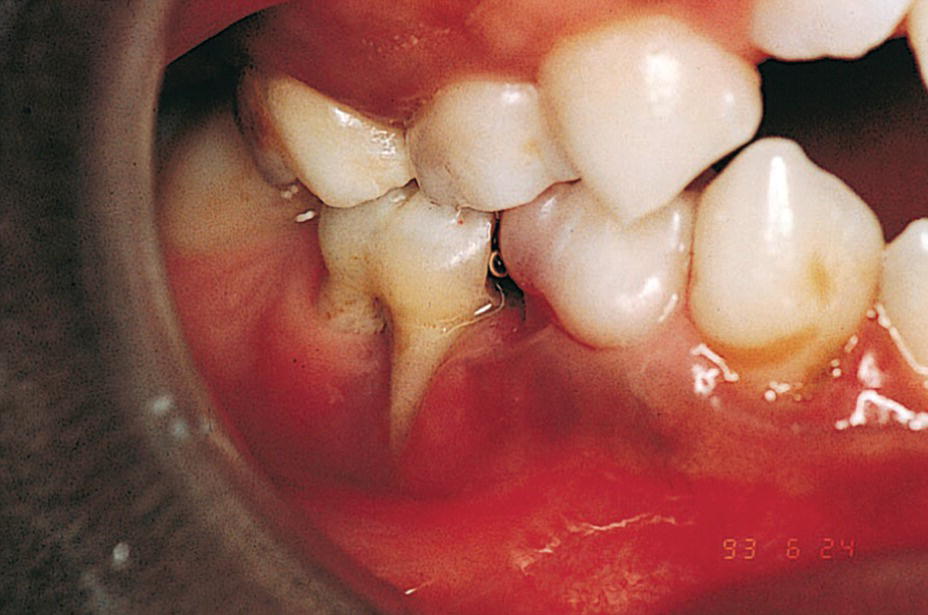
Figure 23.5 A 9‐year‐old boy with a congenital HIV infection exhibiting periodontal disease.
CHCs in children have a variable expression. In order to give the best treatment to a child with such a condition it is important to identify the limitations and also the possibilities of each child. In Box 23.3, 13 different dimensions on a continuous scale used to describe a child with a CHC are presented [2]. It should be observed that the definitions of CHC and impairment, disabilities, and handicap are related. They concentrate on the consequences for the individual rather than on the diagnosis (see Chapter 24, Box 24.1). The international classification of impairments, disabilities, and handicaps model proposes that there are three consequences of disease—impairment, disability, and handicap—‐and that they are sequentially related.
Prevalence
The prevalence of CHCs has been studied in different populations. Depending on the definition, estimates vary from less than 5% to more than 30% of children. The National Health Interview Study [3] used limitations in the kinds or amounts of play activities done by other children (<5 years); needing help with personal care including bathing, dressing, eating, getting in and out of bed and chairs, using the toilet, and getting around at home (3+ years); difficulty walking without equipment (<18 years); difficulty remembering (<18 years); receipt of special education services or early intervention services (<18 years); or any other activity limitation (<18 years). The overall rate of disability for non‐institutionalized children, 18 years old, increased 15.6% between 2001–2002 and 2010–2011 (Box 23.4). The estimated number of children with disabilities increased from 6.9% to 7.9% of the population. In a study of a total population in the south of Sweden in children aged 0–15 years, 8.4% were identified as having a CHC [4]. There is a predominance of boys over girls: 9.2 vs 7.6%. Eight percent of children with CHCs needed extensive help with activities of daily life and/or had a poor prognosis concerning short‐term survival, while 70% had only occasional limitations or needed regular medical treatment which did not interfere with normal activities.
Multi‐handicaps are common, particularly in children with mental retardation who exhibit additional diagnoses such as congenital malformation, epilepsy, vision impairment, and cerebral palsy. Among children with chronic conditions, approximately 70% have one diagnosis, 21% have two, and 9% have three or more. The prevalence of developmental delay, learning disabilities, and emotional and behavioral problems increases sharply with the number of chronic conditions in a child. The number of days in bed, school absences, and activity limitation also increased.
There are strong correlations between social disadvantage and child health. Overall, mortality throughout childhood and adolescence is increased among socially disadvantaged groups. In addition, survival of CHCs is worse in socially disadvantaged children. Factors apart from the material aspects are poorer social support, lifestyle factors such as smoking, poor dietary habits, lack of breastfeeding, and parenting style.
Despite obvious differences among the specific types of health conditions, important commonalties exist in the experience of children and families affected with various conditions. Among them are the need for a wide array of community and professional services, increased challenges to self‐concept and optimal emotional development, extra financial hardship, and disruption of family and social activities (see also Chapter 24).
Increased risk for oral diseases in children with chronic health conditions
Children with CHCs have an increased risk of oral diseases due to consequences of the disease or the medication given. In Box 23.5, groups of children with CHCs who have an increased risk for oral diseases are presented [5]. An important part of the general dentist’s role is to maintain contact with the family and to initiate the necessary preventive measures and to refer the patients if the condition of the patient requires pediatric dentistry specialist care. An early diagnosis of oral diseases is also of major importance as well as knowledge of any changes in health condition and medication that may affect the dental treatment. The medical history should be examined very carefully covering the child’s whole lifespan. It is of particular importance if local anesthesia and sedation are to be used that the child is classified according to anesthetic risk.
Preventive strategies in children with chronic health conditions
There are many CHCs that can directly influence dental care and some are the consequences of dental disease, and also dental treatment may have life‐threatening consequences. Unfortunately, there exist barriers to accessing dental care. Many children and adolescents with CHCs require frequent and sometimes prolonged hospitalizations that separate them from their home environment. This may result in missed appointments. Parents may also be reluctant to once again review the history of the child with the dentist or may not understand the need to share this information. Knowledge and skills among dentists may also not be adequate to treat children with severe CHCs. Early professional intervention is particularly important to provide examination, risk assessment, and information and guidance to parents so that oral diseases can be prevented. Box 23.6 summarizes preventive strategies that can be implemented in children with CHCs.
Chronic health conditions
Prematurity
Preterm is defined by the World Health Organization (WHO) as babies born alive before 37 weeks of pregnancy are completed. Sub‐categories are provided based on the gestational age (GA): moderate to late preterm (32 to < 37 weeks), very preterm (28 to < 32 weeks), and extremely preterm (<28 weeks). Furthermore, preterm children are often categorized according to their birth weight (BW); low birth weight (<2500 g), very low birth weight (<1500 g), and extreme low birth weight (<1000 g). In Western countries, the reduced mortality and morbidity of especially extremely preterm children have increased the prevalence. The survival of preterm birth is, though, not without complications. Immediate complications are, e.g., respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular bleeding, necrotizing enterocolitis, persistent ductus arteriosus, along with increased risk of infections, whereas late complications include cerebral palsy, neuropsychiatric disorders, learning disabilities, altered pain perception, and restricted or delayed growth, among others. Factors that influence the development and growth of a child, prenatally or in the early years of life, are also potential factors to influence the development of the primary and permanent teeth.
Implications for oral health
It is well documented that premature children have an increased risk of developing hypoplasia in the primary dentition. Furthermore, studies suggest an increased risk of enamel opacities in the primary dentition in the case of very low birth weight [6]. Early diagnosis and prevention are therefore important to prevent development of early childhood caries. Furthermore, the extreme preterm children can display behavior management problems, due to hypersensitivity and delayed development, which should be considered when treating these children in the dental clinic.
Asthma
Asthma is one of the CHCs with an increasing prevalence in the Western world. It is a serious global health problem affecting more than 100 million people, and in most countries the prevalence has increased during the past two decades. In Sweden, 5–7% of the population has asthma. Chronic inflammatory mechanisms are important in the development of obstructive symptoms in asthma. Mild symptoms are treated with β2‐agonists and, when symptoms occur more frequently, treatment with inhalation steroids is instituted.
Implications for oral health
Children with asthma have an increased incidence of dental caries, particularly in the permanent dentition, gingivitis, calculus, and dental erosion as well as an altered salivary composition and flow rate [7]. Although there are conflicting results in the literature regarding the effect on caries, most investigators suggest that children are at higher risk due to their pharmacotherapy as well as the duration of the disease. Contributing factors are the medication these children are taking in the form of inhalers and liquid medicine. A large proportion of inhaled drugs, up to 80%, is retained in the oral cavity. Since inhalation powder often contains sugars such as lactose, this may contribute to the increased caries prevalence. It is recommended that children rinse their mouths with water after each steroid inhalation. An increased consumption of sugar‐containing beverages has also been reported.
The use of β2‐agonists is associated with a decreased salivary secretion rate. The mechanism involved is a downregulation of β2‐receptors in the salivary glands, leading to a decreased secretory signal. An increased incidence of gingivitis is also found in asthmatic children. This has been explained by an altered immune response and their tendencies to mouth breathe, particularly during an episode of rhinitis or acute asthmatic attack. Increased levels of calcium and phosphorus in parotid saliva may be associated with higher levels of calculus in asthmatic children. An increased level of erosive tooth loss is also found. A low salivary secretion rate, frequent consumption of acidic drinks, and an increased incidence of gastroesophageal reflux are reportedly associated factors. The dental treatment itself may cause a stress reaction that may precipitate an attack. Children are advised to bring their medication list to the dental office. Nitrous oxide–oxygen sedation should be used in preference to intravenous sedation if sedation is required. Due to increased risk of caries a regular preventive program should be instituted and the salivary secretion rate should be monitored.
Aspirin and other nonsteroidal anti‐inflammatory drugs (NSAIDs) trigger exacerbations in susceptible people with asthma, a condition referred to as NSAID‐exacerbated respiratory disease (NERD). In people with NERD, aspirin inhibits the constitutively active enzyme cyclo‐oxygenase‐1, increasing the levels of arachidonic acid. This in turn increases the levels of proinflammatory cysteinyl leukotrienes and reduces the levels of anti‐inflammatory prostaglandins tipping the balance towards bronchoconstriction in asthma [8].
Cardiovascular disorders
Almost all heart disease in children is congenital in origin, with a birth prevalence of 8 per 1000 live births. The eight most common structural congenital heart diseases (CHDs) account for over 80% of the total; they are ventricular septal defects, patent ductus arteriosus, atrial septal defects, tetralogy of Fallot, pulmonary stenosis, coarctation of the aorta, aortic stenosis, and transposition of the great arteries.
Larger defects are closed surgically in the first years of life, and some defects may require complex surgery and eventually transplantation. Acquired heart diseases such as myocarditis and infective endocarditis (IE) are still a cause of death and disability in children. The incidence of rheumatic fever has fallen sharply. Regarding IE, it is well accepted that there is a real bacteremia association and that oral streptococci cause 50% of all IE. Dental procedures can easily cause bacteremia. It is assumed that after extraction the prevalence is 96.2% after 30 sec, 20% after one hour. Also toothbrushing (40%) and periodontal probing (40%) are possible triggers.
Implications for oral health
Invasive dental procedures such as extractions, scaling, oral surgery, and endodontic treatment are likely to induce a transient bacteremia. Although it has been shown that all procedures involving the gingival tissues, even toothbrushing, induce a bacteremia [9], no evidence was presented in a recent Cochrane review that antibiotic prophylaxis (AP) is either effective or ineffective in preventing IE in people at risk who are about to undergo an invasive dental procedure. It is not clear whether the potential harms and costs of antibiotic administration outweigh any beneficial effect. Ethically, practitioners need to discuss the potential benefits and harms of antibiotic prophylaxis with their patients before a decision is made about administration [10]. Box 23.7 lists conditions where AP is not necessary, if the patient has no additional risk factors, who undergo any dental procedure that involves the gingival tissues and the periapical region of a tooth and for those procedures that perforate the oral mucosa. Consequently invasive dental procedures need AP whereas procedures such as routine anesthetic injections through noninfected tissue, placement of removable prosthetic or orthodontic appliances, shedding of primary teeth and bleeding from trauma to the lips or oral mucosa do not need AP. In a limited patient population, prophylactic antimicrobial therapy should be directed against viridans group streptococci (Box 23.8) [11].

VIDEdental - Online dental courses


