Infections and infestations
Key Points
• Increasing population mobility means that a wide range of infections is seen globally
• Infections are especially common in immunodeficient people
An enormous range of infections is recognized; many are prevalent in the tropics and developing countries, and some are fatal. Global travel and global warming increasingly bring contact with these infections to the rest of the world. Concern has been expressed about more than a dozen potentially fatal infections that appear to be increasing in their geographical range (Box 21.1).
Bacteria, viruses and fungi are common in the external and internal environment, however, and most cause problems only if they secrete noxious substances, become invasive or elicit inappropriate host defence responses. Protection against most bacteria involves largely B cells and plasma cell production of antibodies, together with phagocytes (neutrophils and macrophages). Protection against mycobacteria, viruses and fungi is largely via T lymphocytes.
Emerging Infections
Emerging infections (see also Appendix 21.1 and Ch. 33) may be:
■ a recognized infection spreading to new areas or populations
■ a hitherto known disease that is discovered to be caused by infection
■ a previously unrecognized infection appearing where the habitat is changing (e.g. deforestation)
■ a new infection resulting from change(s) in pre-existing microorganisms
Infection Control
Guidelines to prevent transmission of infections are found at http://www.bda.org/dentists/advice/ba/ic.aspx (accessed 30 September 2013).
Disease Notification
Notification of a number of specified infectious diseases is required in UK under the Public Health (Infectious Diseases) 1988 Act and the Public Health (Control of Diseases) 1984 Act. New (amended) regulations for clinical notifications came into force on 6 April 2010 (Box 21.2). Registered general medical practitioners (GMPs) in England and Wales have ‘a statutory duty to notify a “proper officer” of the Local Authority of suspected cases of certain infectious diseases’ – usually the consultant in communicable disease control (CCDC). The GMP should fill out a notification certificate immediately on diagnosis without waiting for laboratory confirmation and ensure that it reaches the officer within 3 days (telephone if urgent). The proper officers are required weekly to inform the Health Protection Agency (HPA) Centre for Infections (CfI) of the details of each case of each disease that has been notified. As well as notifications of the infectious diseases specified below, the 2010 regulations also require GMPs to notify cases of ‘other infections or of contamination which they believe present, or could present, a significant risk to human health’, e.g. emerging or new infections, or cases of contamination (such as with chemicals or radiation) – particularly if there is a risk of transmission to others. Diagnostic laboratories also have a requirement to notify the HPA of specified causative agents they identify in tests on human samples.
Notification requires completion of the appropriate form, but urgent cases should be notified by telephone as well (certainly within 24 hours of any suspicions arising).
The following details are required:
■ Patient’s name, date of birth, sex and home address with postcode
■ Patient’s National Health Service number
■ Ethnicity (used to monitor health equalities)
■ Occupation, and/or place of work or educational establishment if relevant
■ Current residence (if it is not the home address)
■ Contact details of a parent (for children)
■ The disease or infection, or nature of poisoning/contamination being reported
■ Date of onset of symptoms and date of diagnosis
There is no fee payable for notification.
In Scotland, written notification should be undertaken electronically via the Scottish Care Information (SCI) Gateway (http://www.hps.scot.nhs.uk/publichealthact/NotifiableInfectiousDiseaseData.aspx; accessed 30 September 2013).
Diseases that are notifiable to the local authorities in the UK and USA are shown in Box 21.2. Incubation times are shown in Appendix 21.2.
Further information may be found at: http://www.hpa.org.uk/infections/topics_az/noids/archive.htm (accessed 30 September 2013).
Bacterial Infections
Bacterial infections and therapy are discussed here and in other chapters (Table 21.1) and in Appendices 21.3, 21.4 and 21.5.
Table 21.1
| Bacterial infection | Chapter location |
| Bartonella infections (Rochalimaea) | This chapter |
| Brucellosis | This chapter |
| Chlamydia | Ch. 32 |
| Cholera | This chapter |
| Diphtheria | This chapter |
| Gonorrhoea | Ch. 32 |
| Granuloma inguinale | Ch. 32 |
| Haemophilus | This chapter |
| Legionella | Ch. 15 |
| Leprosy | This chapter |
| Leptospirosis | This chapter |
| Listeria | This chapter |
| Lyme disease | This chapter |
| Meningococci | Ch. 13 |
| Paratyphoid | This chapter |
| Pertussis | This chapter |
| Plague | This chapter |
| Pneumococci | This chapter |
| Q fever | This chapter |
| Rickettsia | This chapter |
| Salmonella | This chapter |
| Staphylococci | This chapter |
| Streptococci | This chapter |
| Syphilis | Ch. 32 |
| Tetanus | This chapter |
| Trichomoniasis | Ch. 32 |
| Tuberculosis | Ch. 15 |
| Tularaemia | This chapter |
| Typhoid | This chapter |
| Yersinia | This chapter |
Bacterial infections are common. Most are transient with few untoward sequelae but some can cause serious, recurrent, disseminated or persistent lesions – especially in immunocompromised persons (particularly in neutropenic patients, those with organ transplants, and those with human immunodeficiency virus/acquired immunodeficiency syndrome [HIV/AIDS]) – or can be life-threatening immediately (e.g. meningococcal meningitis), less immediately (e.g. diphtheria) or in the longer term (e.g. tuberculosis, syphilis).
Bacterial infections are often diagnosed on clinical grounds, supported by smears, culture, testing for immune responses (serology) and, increasingly, examining for nucleic acids.
Antibacterial drugs can often be effective therapy (see Appendix 21.3) but drainage of pus is often more important. Antibiotic resistance is increasingly a serious problem (e.g. Staphylococcus aureus, Clostridium difficile, Mycobacterium tuberculosis) and is encouraged by unwarranted use of antibiotics. Uncommon bacterial infections are shown in Appendix 21.6. Immunization against various bacteria is available and should be taken up (Appendix 21.7).
A wide range of bacterial infections are recognized (see Table 21.1). This chapter discusses odontogenic and orofacial bacterial infections; most cause lesions of limited duration but some are life-threatening. Nosocomial infections (health-care associated infections; HCAIs), tetanus, puncture wounds and bites, and other infections not discussed elsewhere are then summarized.
Orofacial and Odontogenic Bacterial Infections
Periodontal Infections
Abscesses
A gingival abscess may arise from infection or a foreign body. A lateral periodontal abscess (parodontal abscess) is seen almost exclusively in patients with chronic periodontitis but may follow impaction of a foreign body or, rarely, can be related to a lateral root canal on a non-vital tooth.
Clinical features
Erythema and swelling are the main features. Lateral periodontal abscesses may be painful and eventually may discharge – either through the pocket or buccally, but more coronally than a periapical abscess.
General management
Drainage is needed, and sometimes antibiotics (Table 21.2).
Table 21.2
Antimicrobials for odontogenic and antral infectionsa
| Condition | In non-allergic individuals, antimicrobial for>3 days or until symptoms resolve | Comments |
| Acute necrotizing gingivitis | Metronidazole or amoxicillin | Only if systemic involvement |
| Bites | Co-amoxiclav | Ch. 24 |
| Cellulitis | Benzyl penicillin plus flucloxacillin | – |
| Periapical abscess | Amoxicillin or metronidazole for 5 days | Only if systemic involvement or cellulitis |
| Pericoronitis | Metronidazole or amoxicillin | Only if systemic involvement or trismus |
| Periodontal abscess | Amoxicillin or metronidazole for 5 days | Only if systemic involvement or cellulitis |
| Periodontitis | Metronidazole or doxycycline | Only for severe disease |
| Sinusitis | Amoxicillin or doxycycline or erythromycin for 7 days | Only for symptoms>7 days |
Acute necrotizing ulcerative gingivitis
Acute necrotizing ulcerative gingivitis (ANUG) is a non-contagious anaerobic infection associated with proliferation of Borrelia vincentii and fusiform bacteria. It is typically an infection of young adults, found especially in institutions, the armed forces, etc., and predisposing factors include poor oral hygiene, smoking, viral respiratory infections and immune defects such as in HIV/AIDS.
Clinical features
Characteristic features of ANUG include profuse gingival bleeding, severe soreness from gingival ulceration, halitosis and a bad taste. Malaise, fever and cervical lymph node enlargement are rare.
General management
Diagnosis is usually clinical. Smears show fusospirochaetal bacteria and leukocytes. Occasionally, ANUG may be confused with acute leukaemia or herpetic stomatitis, and a full blood picture may be needed. HIV infection may need to be considered.
Management is by oral debridement, metronidazole (penicillin, if pregnant) and improved oral hygiene.
Noma (cancrum oris; gangrenous stomatitis)
Noma can result from ANUG in malnourished, debilitated or immunocompromised patients, especially in children in developing areas. Anaerobes have been implicated, particularly Bacteroides (Porphyromonas) species, Fusobacterium necrophorum (an animal pathogen), Prevotella intermedia, Actinomyces and alpha-haemolytic streptococci. In cases following ANUG, Streptococcus anginosus and Abiotrophia species are the predominant organisms. In early noma, predominant species include Ochrobactrum anthropi, Stenotrophomonas maltophilia, an uncharacterized species of Dialister and an uncultivated phylotype of Leptotrichia. A range of species or phylotypes is found in advanced noma, including Propionibacterium acnes, Staphylococcus species, Stenotrophomonas maltophilia, Ochrobactrum anthropi, Achromobacter species, Afipia species, Brevundimonas diminuta, Capnocytophaga species, Cardiobacterium species, Eikenella corrodens, Fusobacterium species, Gemella haemolysans and Neisseria species. Phylotypes unique to noma infections include those in the genera Eubacterium, Flavobacterium, Kocuria, Microbacterium and Porphyromonas, and the related Streptococcus salivarius and genera Sphingomonas and Treponema. Spreading necrosis penetrates the buccal mucosa, leading to gangrene, an orocutaneous fistula and scarring.
Diagnosis is clinical; an immune defect should always be excluded. Management includes improving nutrition, systemic antibiotics (clindamycin, penicillin, tetracyclines or metronidazole) and plastic surgery.
Pericoronitis
Acute pericoronitis is inflammation of the operculum over an erupting or impacted tooth, usually a mandibular third molar. It appears in relation to the accumulation of plaque and trauma from the opposing tooth. A mixed flora with Fusobacterium and Bacteroides is recognized to be important. Immune defects may predispose.
Clinical features
Acute pericoronitis manifests with pain, trismus, swelling and halitosis. The operculum is swollen, red and often ulcerated, and there may be fever and regional lymphadenitis. Pus usually drains from beneath the operculum but, in a migratory abscess of the buccal sulcus, may track anteriorly.
General management
Diagnosis is from clinical features. Radiology is usually indicated to confirm the position and root formation of the underlying partially erupted tooth.
Initial management comprises local debridement and application of antiseptics such as chlorhexidine. Reduction of the occlusal surface (or extraction) of an opposing tooth may be helpful if there is local trauma. Pyrexia, trismus or cervical lymphadenopathy may be indications for use of systemic antibiotics, typically metronidazole. Long-term treatment may include extraction of the associated impacted tooth, particularly when this is a lower third molar.
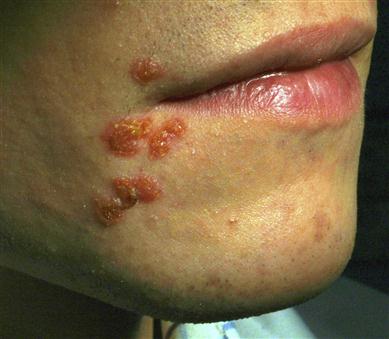
Dental abscess (periapical abscess, odontogenic abscess)
General aspects
A dental abscess is often a sequel of pulpitis caused by dental caries, but may arise in relation to any non-vital tooth. A mixed bacterial flora, especially anaerobes such as Fusobacterium and Bacteroides (Porphyromonas), is implicated.
Clinical features
The causal tooth is non-vital but tender to palpation. Most dental abscesses produce an intraoral swelling, typically on the labial or buccal gingival; those on maxillary lateral incisors and those from palatal roots of the first molar tend to present palatally. Occasionally, abscesses track or discharge elsewhere; for example, lower incisors or molars may discharge extraorally, and maxillary premolars and molars may discharge into the maxillary sinus (Fig. 21.1). Pain and facial swelling are characteristic but, once the abscess discharges, the acute inflammation, pain and swelling resolve and a chronic abscess develops discharging from a sinus – usually buccally and intraorally. Acute periapical suppuration may track through the cortical plate and may be limited by fascial planes within anatomical spaces or spread beyond them, as in the case of Ludwig’s angina. Spread may also be lymphatics to regional lymph nodes, or haematogenously leading to thrombophlebitis, bacteraemia or even septicaemia.
General management
Diagnosis is from clinical features plus imaging. Extraction or endodontic therapy of the affected tooth removes the source of infection. Analgesics may be indicated. Antimicrobials are required only in the circumstances outlined below.
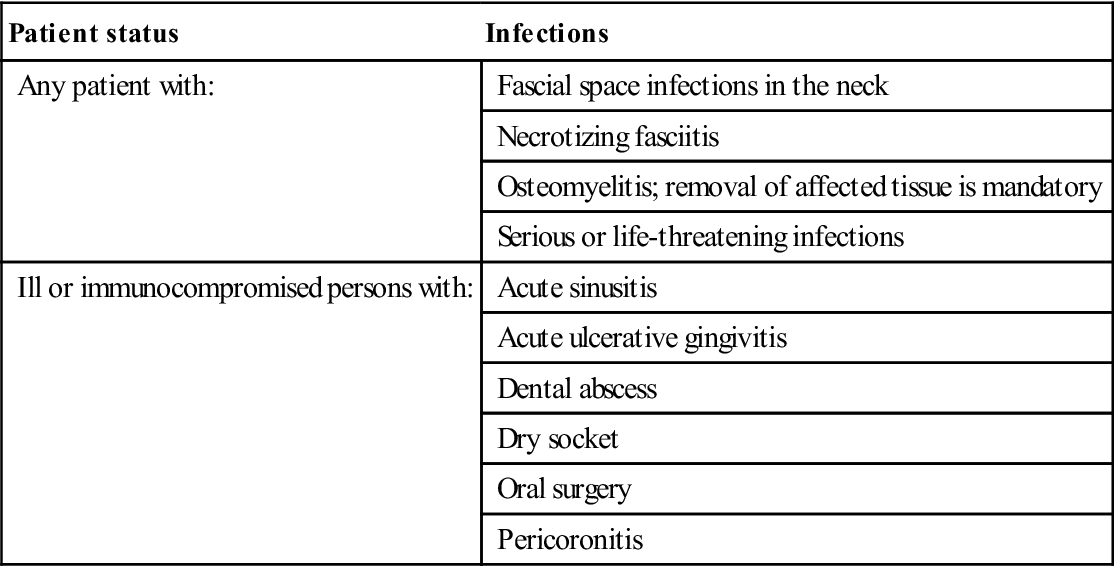
Odontogenic Infections
Odontogenic infections are mainly a consequence of pulpitis that leads initially to periapical infection and a dental abscess. Most odontogenic (and many orofacial) infections arise from the commensal oral mixed flora, with a substantial proportion of anaerobes. Most odontogenic and orofacial infections respond to drainage, by either endodontic treatment, incision or tooth extraction. A drain usually needs to stay in place for 24–48 hours until most/all of the pus has discharged. Analgesics also may be required. Antimicrobials may be indicated in a number of circumstances (Table 21.3).
Table 21.3
Indications for antimicrobial therapy
< ?comst?>
| Patient status | Infections |
| Any patient with: | Fascial space infections in the neck |
| Necrotizing fasciitis | |
| Osteomyelitis; removal of affected tissue is mandatory | |
| Serious or life-threatening infections | |
| Ill or immunocompromised persons with: | Acute sinusitis |
| Acute ulcerative gingivitis | |
| Dental abscess | |
| Dry socket | |
| Oral surgery | |
| Pericoronitis |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Most odontogenic infections respond well to penicillin or metronidazole, but increasing rates of resistance due to production of beta-lactamase (an enzyme that degrades penicillins) have lowered the usefulness of many penicillins. Amoxycillin (±metronidazole) is a common first choice: second choices include cefuroxime, erythromycin, or clindamycin. Co-amoxiclav plus clindamycin are increasingly used first-line because of their broad spectrum of activity and resistance to beta-lactamase.
Anaerobic infections
Most head and neck infections are endogenous and mixed, with anaerobes, two-thirds containing more than one anaerobic species. Predominant anaerobes include Prevotella, Fusobacterium species, Actinomyces species (about 50% are Actinomyces odontolyticus), anaerobic cocci and Eubacterium species. Prevotella intermedia, Fusobacterium nucleatum, Prevotella melaninogenica and the Bacteroides fragilis group are the most common Gram-negative anaerobic species. Microaerophilic streptococci are often associated with anaerobes. Gram-positive anaerobic cocci (GPAC) are detected in about 15% of specimens – Finegoldia magna accounting for about one-third. Among aerobic/facultative isolates are Gram-positive cocci, Gram-negative bacteria and Candida species.
Treatment involves surgical procedures and antibacterial agents, which should cover both aerobes and anaerobes. Resistance rates to some agents (such as ampicillin/sulbactam and clindamycin) have increased.
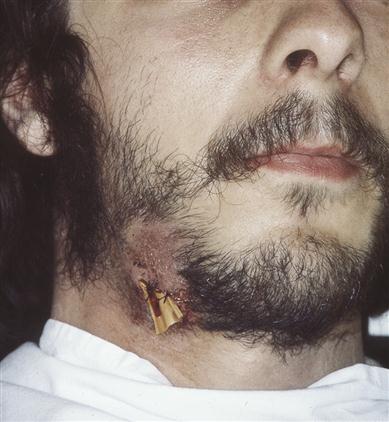
Group A streptococcus (GAS) infections
People may carry group A streptococci in the throat or on the skin without symptoms of illness. Streptococcal oral or head and neck infections are shown in Table 21.4 (see also Fig. 21.2). Most streptococci are highly susceptible to penicillin. Some pneumococci (mostly imported) are increasingly resistant. Few people who come in contact with GAS will develop invasive GAS disease, but people with chronic illnesses like cancer, diabetes and chronic heart or lung disease, and those on immunosuppressive medications such as steroids, have a higher risk. Persons with skin lesions (such as cuts, chickenpox or surgical wounds), the elderly, and adults with a history of alcohol abuse or injection drug use also have a higher risk for disease. Infection with GAS can result in a range of symptoms ranging from mild illness (streptococcal throat or a skin infection such as impetigo) to severe disease (necrotizing fasciitis, streptococcal toxic shock syndrome [STSS]). About 25% of those with necrotizing fasciitis and more than 35% with STSS die (see below).
Table 21.4
Streptococcal infections in the head and neck
| Bacteria | Found in/on | May cause |
| Streptococcus viridans | Normal oral flora | Caries and, rarely, infective endocarditis |
| Strep. pyogenes | Skin and pharynx | Cellulitis, impetigo (Fig. 21.2), necrotizing fasciitis, pharyngitis, scarlet fever or erysipelas, rheumatic fever and carditis |
| Strep. pneumoniae (pneumococci) | Upper respiratory tract | Acute glomerulonephritis, meningitis, sinusitis, otitis media, bronchitis and pneumonia |
Staphylococcal infections
Staphylococcal oral or head and neck infections may be caused by Staphylococcus aureus; most are minor (such as furuncles and boils) and most can be treated without antibiotics, but S. aureus can also cause serious infections such as surgical wound infections, sinusitis, tonsillitis, otitis externa or media, tracheitis, cellulitis, necrotizing fasciitis and toxic shock syndrome (caused by a staphylococcal-produced toxin that has resulted from nasal packing, and by tampon use). Infection is characterized by fever, hypotension, flushing of the skin followed by desquamation, shock and sometimes death.
Up to 80% of the S. aureus isolates in the West are resistant to penicillin, primarily due to production of beta-lactamase, but these will usually respond to lactamase-stable antibiotics such as flucloxacillin and meticillin. Meticillin-resistant S. aureus (MRSA) is resistant to these, however, and often to other antibiotics. Culture is essential to guide treatment of MRSA infections.
Serious Sequelae of Odontogenic or Orofacial Infections
Fatal dental or orofacial infections are rare unless there is an immune defect, but may include progression to mediastinitis, infective endocarditis, necrotizing fasciitis, brain abscess and disseminated intravascular coagulation. Patients with advanced infections need urgent admission for intravenous antibiotics and urgent surgery to remove the cause as well as for incision and drainage of tissue spaces involved. ICU may be needed until the airway is assured. Fibreoptic endotracheal intubation or occasionally emergency surgery (cricothyrotomy or tracheostomy) may be indicated.
Cellulitis
Cellulitis is usually an acute streptococcal or staphylococcal skin infection. It normally resolves on treatment with benzyl penicillin plus flucloxacillin or, if the patient is penicillin-allergic, clarithromycin, erythromycin, clindamycin or vancomycin/teicoplanin. Cellulitis can spread locally or systemically.
Buccal cellulitis is usually caused by Haemophilus influenzae type B, spread by bacteraemia, by lymphatics from, for example, otitis media, or more probably from direct invasion through the oral mucosa. It is an uncommon but distinctive infection, characterized by swelling, tenderness, induration and warmth of the cheek soft tissues in the absence of an adjacent oral or skin lesion; it almost invariably affects children under the age of 5 years. A minority develop meningitis. Blood and cerebrospinal fluid cultures should be taken and treatment with intravenous cefuroxime started.
Lymphangitis
Lymphangitis (inflammation of the lymphatics with pain and systemic symptoms) is commonly secondary to an acute streptococcal or staphylococcal cellulitis, or to an abscess in the skin or soft tissues. Lymphangitis may be confused with thrombophlebitis and suggests that an infection is progressing, and may lead to bacteraemia, septicaemia and life-threatening infection.
Fascial space infections
Fascial space infections of the neck are dangerous, since they can embarrass the airway, erode the carotid vessels, cause toxicity, or spread to the mediastinum or intracranially. They usually arise from the oral flora and are polymicrobial, involving predominantly anaerobes, including Gram-positive cocci and bacilli, as well as Gram-negative bacilli.
Patients with fascial space infections must be admitted for hospital care, which may involve drainage and usually high-dose antibiotics.
Necrotizing fasciitis (Fournier gangrene, Meleney ulcer, postoperative progressive bacterial synergistic gangrene, flesh-eating bacteria, Cullen ulcer)
More information about necrotizing fasciitis is available at: http://www.nnff.org/ (accessed 30 September 2013).
General aspects
Necrotizing fasciitis is a dangerous, rapidly progressive, and spreading infection in the deep fascia, with secondary necrosis of subcutaneous tissues, which destroys muscles, fat and skin. The speed of spread along the deep fascial plane is directly proportional to the thickness of the subcutaneous layer. Most patients are middle-aged or older but, though the condition has become more frequent because of an increase in immunocompromised patients with diabetes, cancer, alcoholism, vascular insufficiencies, transplants, neutropenia or HIV, few have such detectable underlying predisposing factors.
Group A haemolytic streptococci and S. aureus, alone or in synergism, are often the initiating causal bacteria, but other aerobic and anaerobic pathogens, such as Bacteroides (Porphyromonas), Clostridium, Peptostreptococcus, Enterobacteriaceae, coliforms, Proteus, Prevotella, Pseudomonas, Klebsiella, Bacteroides fragilis, Fusobacterium necrophorum and Escherichia coli may be present.
Some men who have sex with men (MSM) have suffered outbreaks of necrotizing fasciitis caused by community-associated MRSA – distinct from health-care-associated strains. Anaerobic streptococci, occasionally seen in drug users, cause many forms of non-clostridial myonecrosis. Necrotizing fasciitis can also be caused by Vibrio vulnificus, often following the consumption of raw seafood – especially in patients with chronic liver disease. There may also be a relationship between the use of non-steroidal anti-inflammatory drugs (NSAIDs) and the development of necrotizing fasciitis during varicella infections.
Clinical features
There is often a history of trauma or recent surgery to the area. Features of necrotizing fasciitis include the following:
■ Early (usually within 24 hours):
Usually a minor trauma or other skin opening (the wound does not necessarily appear infected)
’Flu-like symptoms, such as diarrhoea, nausea, fever, confusion, dizziness, weakness and malaise
■ Advanced (usually within 3–4 days):
Swelling of the limb or area of body experiencing pain, possibly accompanied by a purplish rash
Large, dark marks on the limb, which will become blisters filled with blackish fluid
Necrotic appearance of the wound, with a bluish, white or dark, mottled, flaky surface
■ Critical (usually within 4–5 days):
The lesion begins with an area of thrombosis and skin necrosis, which is initially red, painful and oedematous. Erythema quickly spreads over hours to days, and rapidly turns purplish, dusky and then black, with gas and exudate, and pain disproportionate to the clinical appearance. The margins of the infection move into surrounding skin without being raised or sharply demarcated. Over the next several hours to days, despite severe pain, there may be cutaneous anaesthesia – an unusual combination, as the cutaneous nerves are damaged by the infection (hence anaesthesia) but the proximal stump is irritated (hence the pain). Multiple patches develop to produce a large area of gangrenous skin.
Early on, the patient may look deceptively well but, within 24–48 hours, fever appears with rapidly spreading tissue necrosis, so that the patient usually appears moderately to severely toxic.
General management
Necrotizing fasciitis is uncommon but potentially fatal; if from a dental source, it can also spread and may threaten the airway. The mortality can sometimes reach 30%.
The gas-forming organisms may release subcutaneous gas that may be seen on radiography. Absence of gadolinium contrast enhancement in magnetic resonance imaging (MRI) T1 images reliably detects fascial necrosis. Thoracic computed tomography (CT) may be required to detect mediastinal spread.
Necrotizing fasciitis requires early aggressive treatment. The patient should be admitted to hospital and intubated; the affected area is opened to drain, and necrotic tissue excised. High doses of penicillin or clindamycin are given, plus metronidazole or a cephalosporin, or gentamicin, combined with clindamycin or chloramphenicol. Hyperbaric oxygen, if available, should be given.
Streptococcal toxic shock syndrome (STSS) results in acute hypotension and organ (e.g. kidney, liver, lungs) failure. STSS is not the ‘toxic shock syndrome’, which is due to S. aureus associated with tampon usage.
Recommended therapy for necrotizing fasciitis and STSS is early aggressive surgery plus high-dose antimicrobials (penicillin plus clindamycin). Supportive care in an intensive care unit may also be needed.
Lemierre syndrome (post-anginal septicaemia)
Lemierre syndrome is a rare, potentially fatal, acute anaerobic oropharyngeal infection, the classical presentation of human necrobacillosis. The main pathogen is Fusobacterium necrophorum, an obligate anaerobic, pleomorphic, Gram-negative rod.
The primary infection is in the head in a young, previously healthy person, who subsequently develops persistent high fever and disseminated metastatic abscesses, frequently including septic thrombophlebitis of the internal jugular vein. Lemierre syndrome is often complicated by septic pulmonary emboli and distant metastatic infections.
Surgical drainage and intravenous antibiotics are indicated. F. necrophorum is typically susceptible to penicillin, cephalosporins, metronidazole, clindamycin, tetracyclines and chloramphenicol. Some beta-lactamase–producing strains of F. necrophorum have been reported.
Septicaemia
Septicaemia can arise from odontogenic or orofacial infections, but more commonly from infections of the urinary tract, gallbladder or chest. Immunosuppressed patients are particularly susceptible and oral bacteria are sometimes responsible.
Blood, urine and sputum should be cultured and the patient started on ceftriaxone (a once-daily dose), or cefuroxime plus metronidazole if anaerobic sepsis is suspected.
Actinomycosis (lumpy jaw)
Actinomycosis is a rare chronic infection, usually of the face and neck. It is caused by Actinomyces israelii, a Gram-positive, non-contagious anaerobic bacillus with filamentous growth and mycelia-like colonies bearing a striking resemblance to fungi; it is primarily a commensal found in normal oral cavities, tonsillar crypts, dental plaque and carious teeth. There are three main presentations.
Cervicofacial actinomycosis is the most common and typically causes a red or purplish, somewhat indurated, subcutaneous mass of abscesses and open draining sinuses, usually in the submandibular area near the angle of the mandible, arising a few weeks after an antecedent local lesion (dental or periodontal infection or tooth extraction). Tenderness is slight or absent. Microscopic examination of drained fluid shows ‘sulphur granules’ and Actinomyces, and culture of the fluid or tissue shows Actinomyces species.
Pulmonary actinomycosis causes fever and general malaise, cough and purulent sputum. Cutaneous sinuses may form.
Abdominal actinomycosis may cause pain and a palpable mass in the abdomen.
Treatment of actinomycosis is at least 1–2 months of penicillin or tetracycline. Surgical drainage may be indicated.
Osteomyelitis
See Chapter 16.
Antimicrobial Prophylaxis
Antimicrobial cover may be required for bites; for contact with certain infections (e.g. open tuberculosis, meningitis, Haemophilus influenzae B infections or group A streptococci); and for oral-health-care invasive procedures in people with sickle cell anaemia or asplenia (usually phenoxymethyl penicillin or erythromycin – plus relevant vaccinations). It is sometimes suggested for various procedures or in various other conditions, but otherwise is infrequently indicated (Box 21.3).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses