Chapter 20 Model and investment materials
Learning Objectives
From this chapter, the reader will:
• Be aware of the various materials used in the construction of dental models
• Understand their indications
• Understand the chemistry behind and the properties of gypsum materials
• Be able to correctly manipulate these materials both in the clinic and laboratory
• Be aware of the different types of investment material and each is indicated
• Understand the properties required of an investment material and how to manipulate them to best effect.
Introduction
The last section of this book deals with the materials used in the process of fabrication of indirect restorations and dentures. In all cases the underlying method of production involves a technique that can be traced back many hundreds of years and which was used extensively in the manufacture of jewellery: the lost wax technique. In this technique, a model of the substructure is first prepared. On this the prototype prosthesis is made using materials such as waxes that can be shaped to the required anatomical shape but which can also be destroyed by heating. Once this prototype is prepared it is invested or surrounded in a material which on setting will form the negative of the prototype pattern. The material for the prototype is then removed by heating. This leaves a space in the investing materials, which is filled either by casting or by applying a dough of the material and closing the mould under pressure. The details of this process will be described for each application.
Both the preoperative model and the working cast are constructed out of a material based on gypsum. This is commonly referred to as (dental) plaster. As such, plaster is one of those ubiquitous materials which is used in many types of clinical dentistry and in the dental laboratory. Dental plaster is also traditionally used to make an impression of the edentulous mouth prior to the construction of a complete denture (see Chapter 15). A special form of plaster may be used when metal restorations are to be cast using the lost wax technique. This chapter discusses all the dental materials used in the construction of dental models and those used as investment materials.
Types of Model
• If the resulting model is intended to be used for treatment planning purposes, for example in orthodontics or restorative dentistry, it is known as a study model or study cast.
• A special type of model may be cast for laboratory construction of a restoration. This is referred to as the working cast.
• In the case of a cast restoration such as a crown or bridge, individual teeth may be removed from the rest of the cast so that the restoration can be waxed up and worked on more easily. An individual tooth structure or preparation on a model is known as a die.
There are a range of materials that the technician can use, the choice of which depends on the purpose and use of the cast. In certain cases, for example when the framework for a metal denture is waxed up, the whole model is required. This is called a refractory model and is made out of a special material – a refractory material – so that it may be invested and subjected to high temperature so that the metal framework can be cast on to it. A refractory material retains its shape and strength, that is, it is physically and chemically stable, at high temperatures. This material should also be resistant to thermal shock and have appropriate thermal properties for the intended purpose.
Dental Plaster and Dental Stones
Gypsum is calcium sulphate dihydrate and occurs naturally at many sites around the world. It is crystalline in form (Figure 20.1). To be used as a casting material, the crystalline gypsum is heated at 130°C to remove some of the water contained in it. The product is called plaster of Paris, named after the site where this process was first carried out. The plaster is called calcined and the chemical produced is calcium sulphate hemihydrate. Further heating (up to 200°C) will drive off all of the residual water, leaving behind anhydrous calcium sulphate.
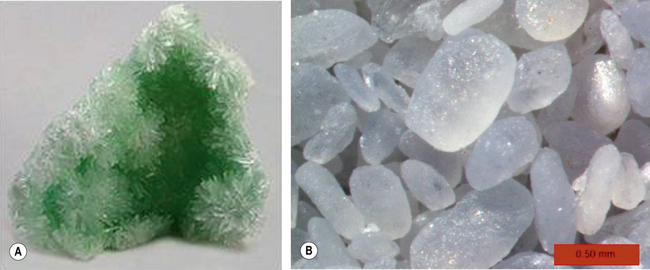
Fig. 20.1 (A) Gypsum stone as it occurs naturally. (B) A micrograph of the microscopic structure of the stone.
Dental modelling plaster
Conventional dental modelling plaster such as plaster of Paris is produced by heating the gypsum to between 110 and 130°C in an open vessel. The hemihydrate so formed is known as the β-hemihydrate. The powder produced is made up of irregular particles which are porous. These particles are not packed closely together (Figure 20.2). Figure 20.3 shows dental models made out of plaster of Paris.
Dental stone
If the dihydrate is heated under pressure and in the presence of water vapour at 125°C, it produces much more uniformly shaped particles. This material has much reduced porosity (Figure 20.4) and is known as α-calcium sulphate hemihydrate. The variant used in dentistry is known as dental stone. Figure 20.5 shows dental models made out of this material.
High-strength dental stone (die stone)
Further treatment of the dihydrate improves the properties of the stone, such as increasing its strength and abrasion resistance. The material produced is called densite, high-strength dental stone or die stone. This material is produced by dehydrating the gypsum in the presence of calcium or magnesium chloride. The combination of chemicals is boiled together, and then the chlorides are washed away with boiling water. The chlorides aid in separating the gypsum particles and the end result is a powder which is even less porous and much less irregular in shape (Figure 20.6). The powder is also the densest of the three types of hemihydrate. Figure 20.7 shows a dental model made out of this material.
Die stones are available in a range of colours (Figure 20.8).
Chemical consituents
Other chemicals may be added to the stone for various reasons:
• Potassium sulphate is added to accelerate the setting time. A 2% solution is used as an alternative to water and will reduce the setting time of model plaster from 8–10 to 4–5 minutes. The compound crystallizes very quickly and encourages further crystal growth. A 4% solution decreases the setting expansion.
• Borax is used to retard the set. A 2% solution will prolong the setting time of some gypsum materials by up to a few hours. The addition leads to the formation of a calcium salt of the borate. This is deposited on the dihydrate crystals, preventing further crystal growth.
• The addition of sodium chloride has the effect of reducing the setting expansion by providing extra sites for crystal growth. This in turn reduces the degree of growth at individual sites so preventing the crystals from being pushed apart.
• Calcium sulphate dihydrate provides nuclei of crystallization and therefore it acts as an accelerator. These set particles have a marked effect when used at very low concentrations between 0.5 and 1%. However, its effects above this value are less apparent.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses