Chapter 20
Dental Professionals as Part of an Interdisciplinary Team
Teresa E. Johnson1, Jayne E. Cernohous2, Paul Mulhausen3, and Deborah A. Jacobi4
1 Apple Tree Dental, Minneapolis, MN, USA
2 Department of Dental Hygiene, Metropolitan State University, St. Paul, MN, USA
3 Telligen, West Des Moines, IA, USA
4 Helping Services for Northeast Iowa, Decorah, IA, USA
Introduction
This chapter is divided into three sections. The first reviews the relationship between oral health and systemic health starting with a brief history of the focal infection theory of disease and then addressing our current understanding of the oral health–overall health connection in older adults, including an overview of a number of oral and medical conditions that have the potential for interaction and association. There is a growing body of literature supporting this important linkage, which emphasizes the importance of an interprofessional healthcare approach to caring for our aging population. The second section addresses the value of an interprofessional team approach to address the physical, medical, dental, psychologic, social, and nutritional needs of older adults. In addition, strategies and opportunities for dental professionals (DPs) to collaborate and engage others in interprofessional care for their elderly patients are included, as well as suggestions for enhancing effective consultations between DPs and other health professionals. The third section of this chapter will present the growing interest in exploring workforce solutions to improve access to care and help meet the dental care needs of the elderly and other age groups. New mid-level providers, with expanded clinical skill sets, can expand the available dental workforce and also participate on the interprofessional care team.
THE ORAL HEALTH–OVERALL HEALTH RELATIONSHIP
Historical retrospective: focal infection theory of disease
The relationship between oral health and overall health is not a new concept. In fact, the relationship between oral and systemic disease has been discussed for more than a century. In an 1891 Dental Cosmos report, American physician and dentist Willoughby D. Miller, who at the time was working in Robert Koch’s laboratory in Berlin, coined the term “focus of infection” and implicated oral microorganisms in the etiology of systemic diseases such as brain abscesses, gastric disorders, and pulmonary diseases (Miller, 1891). Similarly, in 1900, British physician William Hunter published a report attributing oral infections to several systemic diseases such as obscure fevers, anemia, numerous nervous system disturbances, gastritis, colitis, chronic rheumatic infections, and kidney diseases. Later, in 1911, he gave a speech implicating poor dental health and oral infections, brought on by poorly made or ill-fitting dental prostheses, to systemic disease (Barnett, 2006; Pallasch & Wah, 2003; Reimann & Havens, 1940; Rhein, 1912). In the USA, American physician Frank Billings promoted his own theories regarding focus of infection and systemic health affects. In 1909 he reported that 4 of 12 patients with infective endocarditis (IE), whose blood cultures yielded streptococci, had a history of tonsillitis or alveolar abscesses shortly before cardiac symptoms began. He proposed a relationship between endocarditis, bacteremia, and oral focal infections (Gibbons, 1998).
Billings introduced the focal infection theory in 1912. He explained that systemic diseases occurred when bacteria from a focus of infection disseminate through the blood stream or lymphatic system to distant organs. Billings proposed that foci of infection usually occur in the head, with tonsils and teeth particularly vulnerable, since the mouth and airways are subject to frequent microbial exposure (Gibbons, 1998). He attributed oropharyngeal foci of infection to pathologies such as arthritis and nephritis, and advocated tonsillectomies and the extraction of teeth to cure those maladies. With the support of prominent physicians of the time and acceptance within the dental community, the focal infection theory of disease (FITD) lead to the removal of millions of tonsils, adenoids, and teeth over the ensuing years (Pallasch & Wahl, 2003).
This dramatic approach to managing mouth and pharyngeal infections to prevent systemic diseases wasn’t without its detractors in the scientific community. By the 1930s, the popularity of the FITD waned. An influential publication in 1940 questioned the FITD-driven removal of tonsils and teeth, citing several concerns – namely, that the FITD had not been proven and its infectious etiology remained unknown; that individuals often continued to experience symptoms of the original diseases for which their teeth and or tonsils were removed; and that large numbers of individuals having had tonsils removed, were no better off than individuals who retained their tonsils (Pallasch & Wahl, 2003; Reimann & Havens, 1940). In addition to the lack of scientific support for the removal of teeth based on the influences of the FITD, a number of other factors contributed to a decrease in performing extraction of teeth in the second half of the 20th century. These factors included the discovery of penicillin and other antibiotics; advances in diagnosis, treatment and management of periodontal disease as well as increasingly predictable endodontic techniques; improved and expanded restorative options; growing personal preferences to retain teeth; and dental insurance availability.
Despite the de-emphasis of the FITD by the mid-1900s, advances in scientific inquiry and clinical research methods acknowledged situations where oral bacteria could affect distant organs and tissue, such as IE in susceptible individuals (Barnett, 2006). Similar examples would be streptococcal pharyngitis and acute rheumatic fever and in rare cases, orthopedic prosthetic joint infections (OPJIs) or brain abscesses with oral bacteria isolates. A 2003 review of focal infection described IE, brain abscess, and OPJI as the three most documented, publicized, and litigated examples of focal infection (Pallasch & Wahl, 2003).
Current understanding: the mouth–body connection
In recent years there has been renewed interest in the associations between oral and systemic health, which justifies continued advocacy for expanded interprofessional health collaboration among the various health disciplines. Dr. David Satcher, 16th US Surgeon General, when announcing the release of Oral Health In America: A Report of the Surgeon General, said: “In the past half-century, we have come to recognize that the mouth is a mirror of the body, it is a sentinel of disease, and it is critical to overall health and well-being” (US Department of Health and Human Services, 2000). Several oral–systemic health interrelationships are relevant in the management of older adults’ health. There are oral diseases and conditions that influence systemic health as well as systemic diseases and conditions that affect oral health (Griffin et al., 2012). Likewise, treatments used to cure or manage various conditions can affect oral or systemic health, or both. Citing the example of chronic inflammatory diseases to other conditions, Iacopino wrote, “The human body is a single unit composed of related biologic processes such that abnormalities of almost any of its parts have profound effects on other body parts and processes” (Iacopino, 2009).
Oral diseases and conditions known to affect overall health include periodontal disease, poor oral hygiene, tooth loss, and untreated intraoral infections. Intraoral infections can lead to facial and periorbital cellulitis and subsequent brain abscesses, cellulitis within facial planes of the neck compromising the airway, sinusitis, and bacteremia capable of harm at distant sites. The consequences of untreated or poorly managed oral conditions such as dental decay, oral pain, tooth loss, loss of oral function, oral malodor, and esthetically compromised dentition can affect the elderly by way of social stigma, decreased self-confidence, isolation, and depression. The following paragraphs describe a number of conditions that reflect the relationship between oral health and systemic health. These examples illustrate the importance of interprofessional education and collaboration in working to improve the health of our aging population.
Oral and system conditions – interrelationships
Periodontal disease
Periodontal disease has received considerable attention the past two decades as a possible contributing factor to, or as having some association with the following systemic conditions: diabetes, metabolic syndrome, coronary artery disease and atherosclerosis, stroke, and chronic obstructive pulmonary disease. The proposed mechanisms for these associations are complex and beyond the scope of this chapter. (See Chapter 11 for further discussion of periodontal disease.) Yet, it is appreciated that periodontal diseases and systemic conditions share similar risk or modifying factors such as smoking, stress, aging, race or ethnicity, male gender (Li et al., 2000), chronic inflammation, and genetics. Diabetics with periodontal disease have greater difficulty with glycemic control, further complicating the management of this metabolic disease.
Current knowledge suggests that oral inflammatory processes and inflammatory mediators produced in response to periodontal infections are significantly involved in the associations between periodontal disease and cardiovascular disease (CVD), atherosclerosis and stroke. Periodontal bacteria, such as Porphyromonas gingivalis, and bacterial byproducts, such as lipopolysaccharides, travel hematologically and cause harmful effects to heart and blood vessels (Babu & Gomes, 2011). According to the American Dental Association (ADA), investigations have demonstrated an association between periodontal disease, atherosclerotic vascular disease, heart disease, and stroke, but they have not demonstrated a periodontal disease causal relationship for CVD (Lockart et al., 2012). While supporting the ADA’s position, the American Academy of Periodontology (AAP) suggests that this should not decrease concerns about the impact of periodontal diseases on cardiovascular health (AAP, 2012).
Tooth loss and edentulism
While complete edentulism (the loss of all natural teeth) has declined across US age groups over the past several decades, there are socioeconomic, ethnic, age, and state of residence differences in edentulous rates (CDC, 2003; Wu et al., 2012; and see Chapter 1, Aging). In the USA, approximately 25% of adults aged over 65 are edentulous (CDC, 2011). Tooth loss has been linked to heart disease, stroke-related and CVD deaths, atherosclerotic plaque formation in carotid arteries, and angina pectoris; in addition to higher fasting plasma glucose, cholesterol, and blood pressure (Holmlund & Lind, 2012; Lee et al., 2010; Okoro et al., 2005; Watt et al., 2012; Ylöstalo et al., 2006).
Other reported associations between tooth loss and poorer general health include nephropathy, poor oral hygiene, cancer, and neurologic diseases (Tramini et al., 2007). Ten years of longitudinal data of 144 elderly religious women of the School Sisters of Notre Dame, Milwaukee, Wisconsin, ranging in age from 75 to 98 years, demonstrated that those with the fewest teeth had the highest risk of dementia prevalence and incidence (Stein et al., 2007). These religious women belonged to a much larger and still ongoing “Nun Study,” a longitudinal study of Alzheimer’s disease and aging involving 678 American members of the School Sisters of Notre Dame religious congregation.
Associations between edentulism and health outcomes such as malnutrition, poor quality of life, and mortality necessitate interprofessional collaboration. Tooth loss negatively impacts nutrition as fewer natural teeth coincide with decreasing fruit, fiber, dark green and orange vegetable intake, and lower serum levels of beta carotene, folate, and vitamin C (Nowjack-Raymer & Shelham, 2003; Savoca et al., 2010). Persistent vitamin B complex and vitamin C deficiencies result in oral soft tissue changes. Multiple US and international studies have shown a relationship between tooth loss and early mortality while controlling for confounding factors (Anasi et al., 2010; Brown, 2009; Padilha et al., 2008).
Aspiration pneumonia
Aspiration of oropharyngeal bacteria has been shown to cause nosocomial pneumonia in older adults (Russell et al., 1999). Frail elderly residing in long-term care facilities or admitted to the hospital are at considerable risk for aspiration pneumonia (AP) (Pace & McCullough, 2010). El-Solh and colleagues investigated the association between dental plaque colonization and lower respiratory tract infection in hospitalized long-term care elders. For some who developed pneumonia, their dental plaque pathogens matched those isolated from their lungs, implicating dental plaque bacteria and poor oral hygiene to cases of AP (El-Solh et al., 2004). Since AP is a significant cause of morbidity and death in frail elderly (Tada & Miura, 2012), improved oral hygiene may be protective and play an important preventive role (van der Maarel-Wierink et al., 2013). (Editorial Comment: This reinforces the importance of the discussion of oral health care in long-term care facilities, discussed in Chapters 17 and 19.) The use of oral antiseptic agents such as chlorhexidine or povidone-iodine has shown beneficial effects in the prevention of ventilator-associated pneumonia (Labeau et al., 2011). Scannapieco reported that in two studies using either 0.12% chlorhexidine rinses or 0.2% chlorhexidine gel applications twice daily on patients in hospital intensive care units, the incidence of pneumonia was 60% lower than control groups. Scannapieco noted that the association of poor oral health and periodontal disease to community-acquired pneumonia appears to be minimal (Scannapieco, 2006).
Peptic ulcer disease
Oral health status has been linked to peptic ulcer disease. Helicobacter pylori, a spiral gram-negative organism, is involved in the pathogenesis of gastritis as well as peptic and duodenal ulcer disease; however, only a small percentage of persons infected by H. pylori develop gastrointestinal ulcers (Namiot, et al., 2006). The observation of H. pylori in association with dental plaque has implicated the oral environment as one of many potential pathways for H. pylori transmission (Eskandari et al., 2010). There have been conflicting results worldwide as to the relationship between H. pylori found in dental plaque with gastric H. pylori infection, and the uncertainties continue. Navabi et al. (2011) evaluated all published papers since 2000 found through international databases and narrowed the eligible papers to 23 that met specific quality requirements. A meta-analysis of those reports, involving 1861 patients, found that the prevalence of co-infection with gastric and dental plaque H. pylori was 50%; however, the authors conclude insufficient evidence exists to suggest the efficacy of dental treatment and dental plaque control to the prevention of recurrent gastric H. pylori infection (Navabi, et al., 2011). Gebrara et al. (2006) looked for the persistence of H. pylori in the oral cavity after systemic eradication using triple systemic antibiotic therapy in patients positive for gastric H. pylori. They reported that 18 (60%) of the 30 patients with gingivitis or chronic periodontitis who received the antibiotic therapy continued to harbor H. pylori in their mouths. The authors concluded that the mouths of patients with gingivitis or chronic periodontitis who have H. pylori in their stomachs may be reservoirs for the bacteria (Gebrara et al., 2006).
Therapeutics and treatments affecting oral health, systemic health, or both
Unintended adverse drug reactions (ADRs) are known to occur in every organ system in the body and are often mistaken for objective signs of underlying disease (Abdollahi et al., 2008). Dentists administering or prescribing medications should be mindful of ADRs. Drugs interacting with drugs can enhance or diminish the effects of drugs taken alone, thereby causing potential harmful effects. A discussion about the ADRs of most concern in dentistry is available in a recently published review (Becker, 2011). Drug-related problems are common in older adults and cause considerable morbidity (Hajjar et al., 2011), including oral reactions such as xerostomia, opportunistic infections such as Candida albicans, stomatitis, dysgeusia, glossitis, gingival hyperplasia, discolored teeth (Smith & Burtner, 1994), and osteonecrosis of the jaw (Pazianas et al., 2007). Two recent publications provide a comprehensive discussion and listing of drug-induced oral reactions and side effects. Table 20.1 provides an abbreviated summary (Abdollahi et al., 2008; Kalmar et al., 2012). Drug-related oral problems impact seniors’ quality of life, cause pain and discomfort, affect chewing, swallowing and nutritional intake, and diminish oral hygiene efficacy.
Table 20.1 Common oral side effects associated with drugs or drug classes
| Oral manifestations | Drugs/classes of drugs |
| Ulceration and mucositis | NSAIDs (naproxen, salicylates, indomethacin, etc.) Antineoplastics (doxorubicin, methotrexate, 5-fluorouracil, etc.) Propranolol, spironolactone, thiazides, alendronate, phenytoin, captopril, methyldopa, barbiturates, sulfonamides, tetracyclines, etc. |
| Xerostomia | Antidepressants, antipsychotics, anticholinergics, antihypertensives, antihistamines, decongestants |
| Gingival enlargement | Calcium channel blockers (diltiazem, amlodipine, bepridil, nifedipine, verapamil, etc.) Dihydropyridines (bleomycin) Cyclosporine, sodium valproate, phenytoin |
| Pigmenation | Antimalarials (chloroquine, hydrochloroquine, quinidine, etc.) Tranquilizers (chlorpromazine) Amiodarone, busulfan, clofazimine, estrogen, ketoconazole, minocycline, zidovudine, etc. |
| Swelling | Ace inhibitors, penicillins, sulfa drugs, aspirin |
| Vesiculobullus or ulcerative lesions | Lichen planus-like: Antimalarials, arsenicals, beta-blockers Several other drugs such as: allopurinol, furosemide, chlorothiazide, methyldopa, lorazepam, cimetidine, dapsone, propranolol, phenothiazines, spironolactone, sulfonylureas, tetracycline, tolbutamide, lithium, and many more Erythema multiforme-like: Lupus erythematosus-like: Pemphigoid-like: Pemphigus-like: |
aTable created from information provided by Kalmar et al., 2012.
NSAIDs, nonsterodial anti-inflammatory drugs.
Drug-induced salivary hypofunction
Salivary hypofunction resulting in dry mouth or “xerostomia” is not caused by aging per se; rather, it is an age-associated acquired oral phenomena. Xerostomia in older adults is attributed primarily to the effects of polypharmacy associated with the management of multiple chronic illnesses, and attributed less so, to various systemic diseases. There are more than 500 medications associated with xerostomia (Kalmar et al., 2012). In addition to the subjective symptom of dry mouth, xerostomia negatively impacts oral health and increases oral disease risks (Moore & Guggenheimer, 2008). As discussed elsewhere in this book (Chapter 14, Xerostomia), problems associated with xerostomia in the elderly include root and coronal caries, periodontal disease, gingival inflammation, decreased debris clearance, difficulties with food bolus preparation and swallowing, susceptibility to denture sores and stomatitis, among others. Figure 20.1 illustrates dry mouth in an elderly woman with concomitant coronal and root caries.
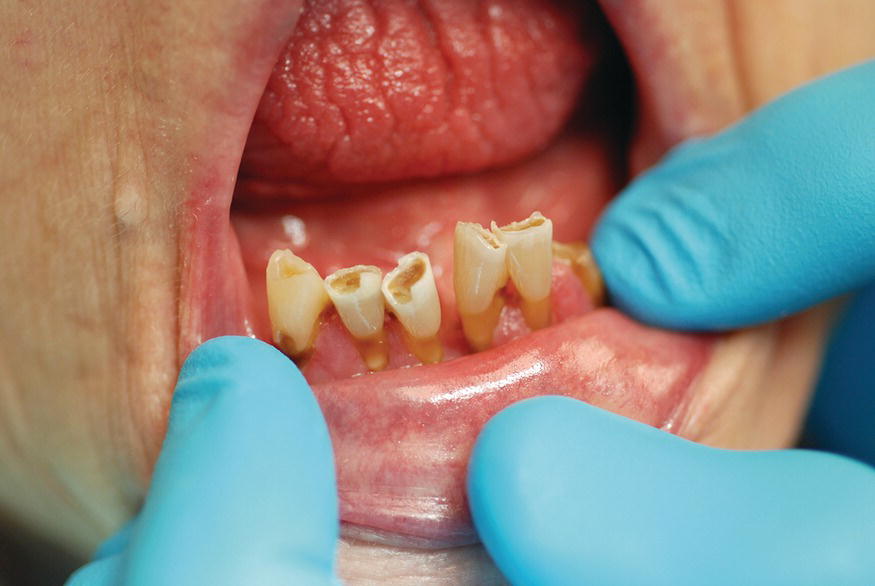
Figure 20.1 Clinical manifestations of xerostomia. Rampant root caries and coronal caries resulting in unsupported and fractured enamel of mandibular anterior teeth. Note the dry, fissured tongue in the background.
Photograph courtesy of Teresa E. Johnson, DDS.
Herbal supplements
While not considered drugs by the US Food and Drug Administration (Meredith, 2001), herbal supplements, such as St. John’s wort, are associated with xerostomia. Other oral manifestations attributed to various herbs include gingival bleeding (feverfew, ginkgo); aphthous ulcers, lip and tongue irritation, and swelling (feverfew); oral and lingual dyskinesia (kava); numbness of tongue (echinacea); and hypersalivation (yohimbe) (Abebe, 2003). Herbal supplements interact with other drugs by altering inflammatory and immune responses, interacting with blood clotting processes and altering enzymatic drug metabolizing activities (Meredith, 2001).
Zinc-containing denture adhesives
Dental professionals should educate their denture-wearing patients about the potential problems associated with zinc-containing denture adhesives, and remind patients to avoid or minimize their use. Excessive zinc is known to cause copper deficiency myelopathy affecting walking and balance, widespread sensory and motor neuropathies, anemia, and bone marrow depression (Crown & May, 2012; Doherty et al., 2011; Nations et al., 2008; Tezvergil-Mutluay et al., 2010). The effects of zinc in denture adhesives should be communicated to other health professionals so that, if denture-wearing patients present to healthcare providers with these symptoms and conditions, the possibility of zinc toxicity from denture adhesives can be considered among differential diagnoses.
Head and neck radiation therapy
Radiation therapy to treat head and neck cancer can result in limited mouth opening and reduced oral motor function, diminished salivary gland function, and subsequent xerostomia and oral mucositis. Mucositis, consisting of erythematous and ulcerative lesions, is an unavoidable and undesirable effect of radiotherapy, and causes pain, dysphasia and decreased oral intake that adversely affects nutrition and quality of life, and contributes to local and systemic infections. The most severe cases of radiation-induced oral mucositis are associated with radiation to primary tumors in the oral cavity and oro- or nasopharynx. It can manifest with concomitant radiation and chemotherapy, or when the total radiation dose is greater than 5000 centigray (Kumar et al., 2009).
Antiresorptive drugs and osteonecrosis of the jaw
Antiresorptive agents, such as bisphosphonates, and denosumab, a nonbisphosphonate antiresorptive agent, are prescribed to strengthen bones and prevent bone fractures in susceptible individuals, especially those with osteoporosis. Long-term use of bisphosphonates is associated with diminished blood supply to the jaw and subsequent development of osteonecrosis of the jaw (ONJ). It has also been called “bisphosphonate-associated osteonecrosis” or “bisphosphonate-related ONJ.” A 2010 report described a 65-year-old woman who developed ONJ while receiving denosumab for the management of a giant cell tumor (Aghaloo et al., 2010). Subsequently, the ADA Council on Scientific Affairs recommended the term “antiresorptive agent-induced osteonecrosis of the jaw” (ARONJ) since it encompasses both bisphosphonate and denosumab-associated ONJ (Hellstein et al., 2011).
Bisphosphonates are prescribed to prevent and treat conditions associated with bone fragility such as osteoporosis, osteitis deformans (Paget’s disease), bone metastasis, and multiple myeloma. Given to arrest bone loss, increase bone density and decrease the risk of pathologic fracture resulting from progressive bone loss, bisphosphonates prevent or slow the loss of bone mass by inhibiting osteoclasts, the bone destroying cells (Little et al., 2008). Denosumab, a human monoclonal antibody, interferes with osteoclast activation and diminishes osteoclast activity. Unlike bisphosphonates, which accumulate in the mineralized bone matrix to an extent reflective of the duration and type of therapy, denosumab does not incorporate into bone and has a substantially lower terminal half-life (Adler & Gill, 2011). Denosumab is administered to treat osteoporosis in postmenopausal women at high risk of bone fractures and to prevent skeletal fractures in persons with bone metastases (Hellstein et al., 2011).
Agent-induced osteonecrosis of the jaw may occur spontaneously or develop when dental conditions or procedures likely to cause trauma to alveolar bone occur. Most commonly, ARONJ is associated with invasive procedures affecting the alveolar bone such as tooth extractions (Hellstein et al., 2011). The exact mechanisms leading to ARONJ remains unknown, but seem to result from the interchange of bone metabolism, local trauma, heightened demand for bone repair, infection, and hypovascularity (Little et al., 2008). ARONJ is associated with discomfort, local inflammation, and possible infection, including a potentially deleterious impact on quality of life. In most cases it never resolves and is managed palliatively or with antimicrobial therapy. In severe cases, jaw fractures occur or jaw resection may be indicated. Therefore, DPs must encourage older adults to achieve optimal oral health before starting antiresorptive therapy whenever possible through patient education and interprofessional communication with physicians, geriatricians and oncologists. It is imperative that DPs’ health history forms include questions on antiresorptive drug use to identify patients at risk for ARONJ.
Systemic diseases affecting oral health
Systemic conditions of the elderly that purportedly influence oral health status include diabetes, neurodegenerative diseases, osteoporosis, acquired autoimmune diseases, and depression. Uncontrolled diabetes affects oral health by exacerbating periodontal infections, interfering with wound healing, and diminishing intraoral pain perception (Saini et al., 2011). The neuromuscular deficits associated with neurodegenerative diseases such as Parkinson’s disease (PD) and multiple sclerosis make it difficult to maintain plaque control and oral health; and the medications to treat them diminish saliva flow (Fiske et al., 2002; Friedlander et al., 2009). Individuals with PD experience decreased oral proprioception affecting occlusion, chewing, and food clearance as well as adaptability to removable prostheses (Friedlander et al., 2009).
Osteoporosis is known to affect alveolar bone height in edentulous patients (Hildebolt, 1997; Kossioni & Dontas, 2007). Autoimmune diseases such as lupus erythematosus and Sjögren’s syndrome are associated with dry mouth, oral mucosal lesions, and dental caries (Brennan et al., 2005; Nazmul-Hossain et al., 2011). Depression can result in poorer oral health due to lack of motivation to carry out routine and effective oral hygiene care, and medications to treat depression are known to cause dry mouth (McFarland, 2010). Gastroesophageal reflux disease is associated with dental caries and tooth erosion due to repeated exposure to acidic gastric contents. Erosion over time can result in poor esthetics, sharp teeth likely to cause mucosal ulcerations, dentinal hypersensitivity, and changes in occlusion and vertical dimension (Barron et al., 2003; Lackey & Barth, 2003; Ranijitkar et al., 2012).
Diagnostic uses of oral fluids
This discussion of the mouth–body connection would not be complete without some attention to “the mouth as a window to general health,” and the known diagnostic capabilities the secretions of the mouth offer for systemic as well as oral disease detection. Currently available and highly accurate salivary diagnostic tests include various hormonal, human immunodeficiency virus (HIV), and alcohol tests. Studies are underway on salivary biomarkers for early oral cancer detection and salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome (National Institute of Health, 2010). Even hepatitis B surface antigen (HBsAg) is detectable in the saliva of hepatitis B-infected individuals with approximately a 75% level of sensitivity (Arora et al., 2012). Gingival crevicular fluid is found to contain diagnostic markers for active periodontal disease (Koregol et al., 2011). The endless possibilities the future holds for saliva and gingival crevicular fluids as widely used tools for systemic disease diagnosis provides optimism that one day innumerable diseases will be diagnosed in an efficient and noninvasive way through easily obtained saliva and crevicular fluid sampling. Certainly, the less invasive the testing, the less stress and risk is placed upon older adults – in particular, the most vulnerable, frail elderly.
INTERPROFESSIONAL CARE
Dental professionals best serve their older patients when they provide dental care as part of a comprehensive healthcare strategy. This care should be patient-centered (Hellyer, 2011) and delivered in partnership with a collaborative interprofessional healthcare team across the healthcare system (Reuben, 2009). Interprofessional teams are widely considered to be essential to the delivery of quality geriatric care (Tsukuda, 1990; Ham, 2002). Well-functioning team care has been shown to have positive effects on patients’ health and it results in better clinical outcomes, higher patient satisfaction, and enhanced delivery of care (Grumbach & Bodenheimer, 2004).
In the care of the older patient, the DP has the greatest impact when working with an expanded interprofessional team that may include physicians, nurse practitioners, physician assistants, nurses, social workers, pharmacists, rehabilitation specialists, and nursing assistants (Coleman, 2005; Polverini, 2012). Additionally, the now-established relationship between oral health and systemic health highlights the need for integrating oral health care into the management of general health care by multiple healthcare providers (Albert et al., 2012; Allen et al., 2008; Iacopino, 2008). While the model of interprofessional care may not be necessary for all geriatric patients, the frequent visits they make to other practitioners present opportunities for promoting oral health (Mouradian & Corbin, 2003). Because oral and systemic health are so closely intertwined in old age, and barriers to dental care are common among the elderly, interprofessional, team-based approaches to care can improve oral health and general well-being. Working as a member of an interprofessional team, DPs maximize their ability to work with other health professionals to assess, diagnose, coordinate, and deliver care to their older patients (Institute of Medicine and National Research Council, 2011).
Because many older patients have a complex set of dental, medical, and social needs, the DP must be able to coordinate their care, respond to these multiple patient needs, and deliver care across many different settings. Providing this care requires effective communication across the locations of care and the disciplines that make up the interprofessional team (Dyer et al., 2004; Institute of Medicine, 2003; Keough et al., 2002; Williams et al., 2002). For example, implementation of a dental treatment plan for a nursing home resident may require coordination with the patient’s medical provider to adjust and manage medications; communication with a licensed nurse to provide treatments, monitoring, and assessment; and delivery of basic oral care by a direct care worker. A social worker may help the patient with transportation needs and payment mechanisms, a pharmacist may provide consultative input into the medication regimen, and family members will have an interest in recommended interventions. By understanding other disciplines and interprofessional collaboration, the DP maximizes the potential to successfully optimize the oral health and well-being of the patient. Table 20.2 lists various patient conditions and the interprofessional care members DPs can collaborate with.
Table 20.2 Potential interprofessional collaboration for dental professionals
| Condition | Association | Potential collaborations with other health professionals | |
| A1C control | Important element of diabetes management |
|
|
| Loss of fine motor skills | Arthritis, stroke, neurodegenerative diseases, trauma, etc. |
|
|
| Dysphagia | Stroke, Parkinson’s, cancer treatment, etc. |
|
|
| Edentulism | Compromised ability to chew, inadequate nutrition |
|
|
| Mucositis / glossitis | Erythematous and ulcerative lesions secondary to cancer tx, drug reactions, etc. |
|
|
| Xerostomia | Increased susceptibility to tooth decay, swallowing difficulties |
|
|
Dental–medical collaboration
DPs can establish successful collaborations with medical colleagues to provide higher quality treatment and better oral health care to their shared patients. Since older patients are more likely to see a primary care medical provider (physicians, physician assistants, and nurse practitioners) than a DP, a more effective integration of medical colleagues into the spectrum of oral health care could have a large impact (Institute of Medicine and National Research Council, 2011). A number of successful dental–medical collaborations have been described in both the dental and the medical literature (Mouradian et al., 2004; Rozier et al., 2003). Interprofessional collaboration implies a higher degree of interaction and coordination than a typical consultation. In the collaborative model, the DP and medical provider integrate their observations, fields of expertise and areas of decision-making in a collaborative and coordinated way to optimize care (Institute of Medicine, 2003). Fundamental to an effective working collaboration, both DPs and medical providers must commit to a shared responsibility for patient care in a climate of mutual respect and trust (Williams et al., 2006; Xyrichis & Lowton, 2008). They should also have knowledge of, and respect for the competences, roles and contributions of other professionals on the team, without any prejudice or stereotyped perceptions (Vyt, 2008).
The ability to communicate and share information efficiently is also essential to successful collaboration. The DP may find that modern communication technology and the use of shared electronic health records facilitate collaboration. Unfortunately, both DPs and medical providers work in fast-paced, interruptive healthcare settings where face-to-face interprofessional collaboration is rare and the absence of this personal contact may prove to be a barrier to effective communication and establishing effective interprofessional relationships (Rice et al., 2010). Perceived busyness is often reported as a barrier to interprofessional collaboration. In the absence of programmatic linkages between providers, clinicians often work in parallel, rather than collaboratively (Stille et al., 2005).
Dental–rehabilitation collaboration
With age, many persons neglect their oral hygiene because of diminished motivation and/or impaired function. The elderly suffer from a heavy burden of chronic medical problems, including dementia, arthritis, paralysis due to stroke, and Parkinson’s disease. They may have impaired vision, diminished sense of touch, or poor hand function (Bellomo et al., 2005; Padilha et al., 2007). In these circumstances, the DP should seek productive collaboration with members of the rehabilitation disciplines. It/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


