Dental Materials
Restorative materials used in pediatric restorative dentistry are commonly the same as those used in restorative dentistry in general. This chapter is intended to identify commonly used materials in pediatric dentistry and provide information that applies specifically to their use. Many materials are available, and in many cases, clinical considerations will dictate the choice of the appropriate material. Table 20-1 identifies the most commonly used materials in pediatric restorative dentistry and the relevant clinical considerations. Chapters 21, 32, and 39 will discuss the specific clinical uses of dental restorative materials.
Bases and Liners
Calcium Hydroxide
Calcium hydroxide cements are supplied in a visible light–cured system (Figure 20-1, A) and a two-paste system (Figure 20-1, B). A catalyst paste containing calcium hydroxide, zinc oxide, and zinc stearate in ethylene toluene sulfonamide reacts with a base paste containing calcium tungstate, calcium phosphate, and zinc oxide in glycol salicylate to form an amorphous calcium disalicylate. The alkaline pH aids in preventing bacterial invasion. Studies have shown that calcium hydroxide “softens” under amalgam and resin-based composite restorations.1,2 The results are attributed to hydrolysis of the calcium hydroxide by fluid contamination from dentinal tubules and microleakage. As hydrolysis occurs, occlusal forces cause gingival displacement of the restoration, leading to discrepancies and breakdown at the restoration margin. Visible light–cured calcium hydroxide preparations have demonstrated clinical success3 and may be less susceptible to hydrolysis. When calcium hydroxide is used, a less soluble high-strength base may be placed to overlie the calcium hydroxide.
Zinc Oxide–Eugenol
Zinc oxide–eugenol cement (Figure 20-2) contains zinc oxide, rosin, and zinc acetate in the powder. The rosin increases fracture resistance and the zinc acetate is effective in accelerating the reaction rate. The liquid is a preparation of eugenol, which reacts with the powder to form an amorphous chelate of zinc eugenolate. The zinc oxide–eugenol cements are used to provide a sedative effect, but their low compressive strength presents clinical limitations.
To strengthen zinc oxide–eugenol cements, acrylic resin and alumina reinforcers have been added. Although these cements are stronger, they remain weaker than the zinc phosphate and glass ionomer cements. When evaluated as a base, zinc oxide–eugenol demonstrated significant microleakage in comparison with glass ionomer cement.4 Because of their sedative effects and years of clinical success, zinc oxide–eugenol remains the material of choice for the pulp chamber filling material, following pulpotomies or pulpectomies in the primary dentition. Zinc oxide–eugenol cements should be used with caution under resin-based composite restorations because the eugenol can inhibit the polymerization of the resin. A glass ionomer cement base may be placed over zinc oxide–eugenol, before the placement of resin-based composite, to avoid polymerization concerns.
Zinc Phosphate
Zinc phosphate cement (Figure 20-3) is composed of zinc oxide and magnesium oxide powder that is mixed with a solution of phosphoric acid and water. The zinc oxide alkaline surface reacts with the phosphoric acid to form a cement of zinc phosphate surrounding particles of zinc oxide. The addition of aluminum and zinc ions buffers the solution to slow the setting reaction. Mixing the cement on a cooled slab minimizes heat production during the chemical setting and increases the working time. When using zinc phosphate cement as a base, additional zinc oxide powder is placed in the mixture to thicken it, which increases the strength and decreases the hardening time of the cement.
Glass Ionomer Cement
Glass ionomer cement (Figure 20-4) has become a commonly used basing agent. It has the ability to create a physicochemical bond to tooth structure and to release fluoride. Glass ionomer cement consists of calcium aluminosilicate glass particles mixed with polyacrylic acid. The initial reaction stage involves the ionization of polyacrylic acid, which leads to a change in the polymer chains from a coiled to linear form. The hydrogen ions produced by ionization attack the calcium aluminosilicate glass, which also contains fluoride, and causes the release of metal and fluoride ions. The majority of the metal cations (Ca2+, Al3+), divalent or trivalent, respectively, are bound by the ionized polymer to form cross-linked salt bridges. Calcium and aluminum ions bind to polyacrylic acid at the carboxyl groups, and a gel phase is precipitated to form a matrix of the hardening cement. Calcium carboxylates are formed first as a firm gel because of the rapid binding of calcium to the polyacrylic acid chains. This initial set has the property of being carvable, but at this stage the ionomer is very susceptible to water absorption. Likewise, the free aluminum ions are susceptible to diffusion from moisture contamination and thus are lost from the cement because they are unable to cross-link with the polyacrylic acid chains. Isolation of prepared teeth with a rubber dam is recommended. Aluminum salt bridges are then formed with the polyacrylic acid matrix, and the cement hardens. The trivalent aluminum ions ensure a much stronger cross-linking than is possible with the calcium divalent bonds alone. The slower reaction of aluminum ions is attributed to the more stringent steric requirements imposed by a trivalent ion on a polyanion chain configuration.
Studies have shown that glass ionomer bases and liners exhibit less marginal microleakage than zinc oxide–eugenol, zinc phosphate, and calcium hydroxide,4,5 thereby preventing bacterial penetration. Fluoride is released from glass ionomer cement by dissolution and diffusion. Glass ionomer bases and liners have demonstrated the inhibition of secondary caries formation.6–9 The fluoride released is taken up by both the enamel and dentin adjacent to the material.10–13 This fluoride aids in creating an inhibition zone that is not susceptible to demineralization, when compared with areas adjacent to non–fluoride-releasing materials.14,15
Resin-modified glass ionomer cement preparations are available and can be light cured.16 Photoinitiated polymers have been placed in the glass ionomer cement formulation to provide light polymerization. Although these resin-modified glass ionomer cements can be light cured, the material sets as a true cement, with an acid-base reaction taking place; therefore the material will chemically set without light curing.
Cavity Varnishes
Cavity varnishes (Figure 20-5) are resins that are insoluble in oral fluids. Varnish is to be used with amalgam restorations; it inhibits surface bonding of resin-based composite systems, dentin-bonding systems, and glass ionomer systems as well as prevents the fluoride released from glass ionomer from gaining access to the restoration and tooth interface. The purpose of cavity varnishes is to reduce microleakage at restoration margins and inhibit penetration of corrosion products from amalgam into dentin, thereby preventing tooth discoloration adjacent to restorations. The varnishes do not prevent thermal sensitivity.
Dentin-Bonding Agents
Removing the smear layer was found to increase the effectiveness of the dentin-bonding agents. The newer bonding agents include conditioning or primer components that remove or alter the smear layer over the dentin. This results in the creation of a mechanical bond by the infiltration of monomers into a zone of demineralized dentin, where the monomers polymerize and interlock with the dentin matrix.17 A majority of the contemporary dentin-bonding agents are similar to those previously discussed or composed of 4-methacryloxyethyl trimellitic anhydride.
Self-etching bonding systems (Figure 20-6) have been developed to offer the convenience of etching and bonding simultaneously. Although research demonstrates excellent bonds to dentin and enamel, caution is advised. The products must be used as instructed by the manufacturer, particularly focusing on agitation of the product during placement and the recommended length of application time.18,19
Restorative Materials
Amalgam
Traditionally, amalgam was the material of choice for class I and class II restorations. Today amalgam continues to be an effective restorative material.20,21 A 3-year study of the clinical performance of 260 amalgam restorations (86.4% class II) demonstrated 254 to be successful.22 It is important to understand the clinical makeup and setting reaction of amalgam to correlate restoration successes and failures with the fundamental properties of the material.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 Outline
Outline TABLE 20-1
TABLE 20-1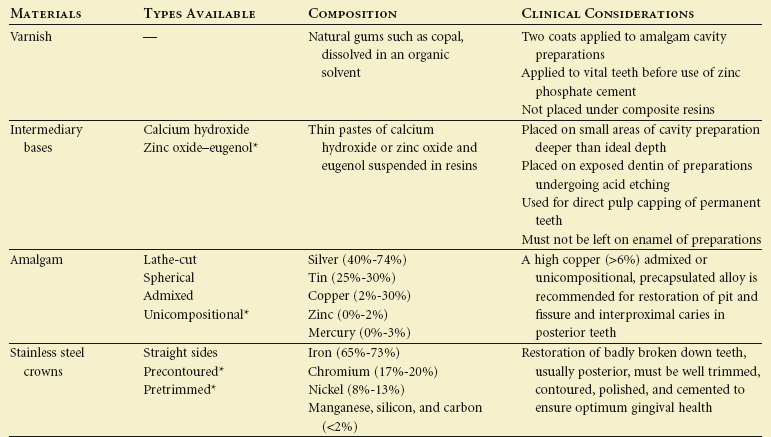
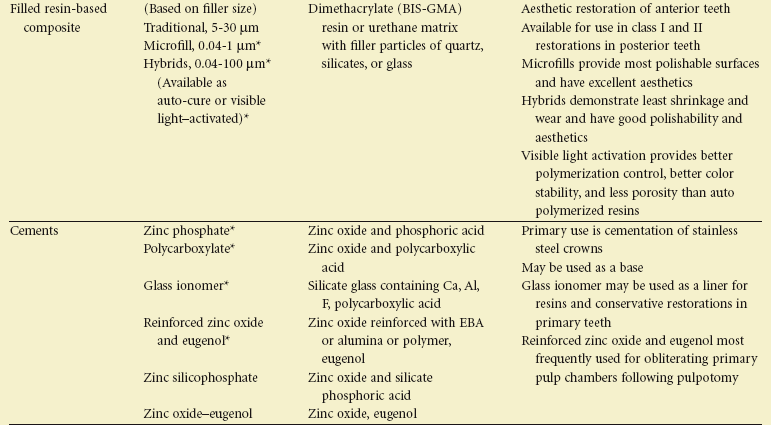
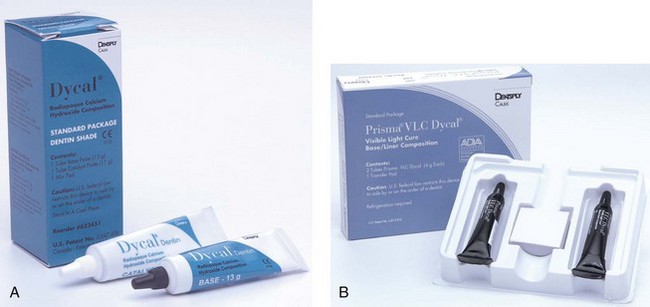
 FIGURE 20-1
FIGURE 20-1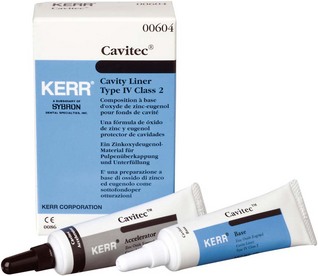
 FIGURE 20-2
FIGURE 20-2
 FIGURE 20-3
FIGURE 20-3
 FIGURE 20-4
FIGURE 20-4
 FIGURE 20-5
FIGURE 20-5
 FIGURE 20-6
FIGURE 20-6