Evaluation and Management of Frontal Sinus Injuries
Development, Anatomy, and Function
The craniofacial skeleton shows evidence of intramembranous ossification of the nasal and frontal bones at 50 to 60 days’ gestation.1,2 By 4 months in utero, the earliest signs of frontal sinus development are present as the middle meatus begins to expand superiorly, creating an early frontal recess.3 At this intranasal location, it is common for numerous furrows to form that will eventually evaginate into the frontal and ethmoid bones.4 One or more of these frontal recess furrows most often pneumatize the frontal bone to become the developing frontal sinus. Alternatively, ethmoid infundibular cells may provide the source of pneumatization that leads to sinus formation; more rarely, the frontal recess may propagate directly into the frontal bone.5 Additional accessory furrows of the frontal recess are important for the development of agger nasi cells and anterior ethmoid air cells. Some of these remain modest in size and contained within the ethmoid while others expand considerably without this confinement.
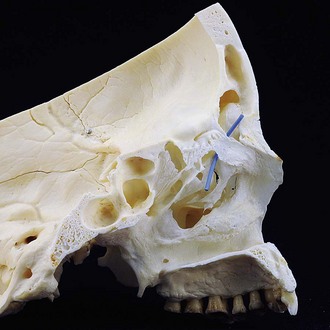
The developmental complexity and heterogeneity of the frontal sinus and anterior ethmoid air cells has the secondary effect of creating a highly variable nasofrontal drainage system. In as many as 80% to 85% of the population, no discernible duct is present and an ostium-type outflow tract serves to drain the sinus.6,7 Alternatively, relative compression of the proximal part of the frontal sinus by developing ethmoid cells may create a true ductal drainage system. When a duct is present, it may vary from 1 to 20 mm in length and 1 to 6 mm in width.8 Drainage into the nose will typically occur below the middle turbinate near the middle meatus, bearing a variable relationship to the ethmoid infundibulum based on the sinus origin (Fig. 19-1). Usually, drainage is directly into the frontal recess and medial to the uncinate process, but less commonly this may occur above or into the ethmoid infundibulum lateral to the uncinate, with rare occurrences above the ethmoid bulla.9,10
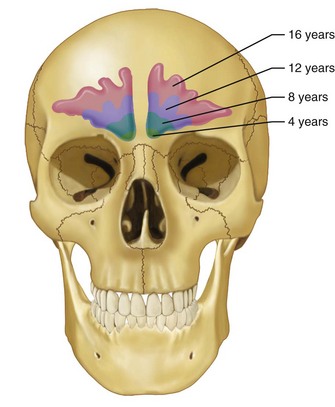
Over time, the frontal sinus pneumatizes to a highly variable degree. Underdevelopment of the frontal sinus is relatively common, with an estimated 4% of the population showing no development and another 5% demonstrating minimal pneumatization.11 Approximately 10% of the population has only unilateral development of the frontal sinus.3 When present, the sinus is not typically identifiable radiographically before the age of 6 years and does not show appreciable development until puberty.12 Size consistent with that of the adult is normally attained by the mid to late teen years (Fig. 19-2).
The fully developed frontal sinus is expected to be highly variable in size and shape. When present, asymmetry between right and left is the norm and bony septations may be present. The sinuses may be present in duplicate or triplicate bilaterally. In general, the sinus is rather diminutive, with a capacity of between 5 and 16 mL.4,12 The average height of a developed frontal sinus is 32 mm and the average width is 26 mm.13,14
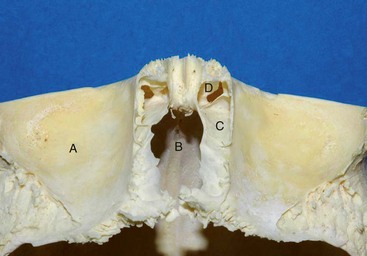
The proximity of the frontal sinus to important anatomic structures accentuates the importance of thoughtful management of these injuries. The anterior portion of the sinus borders the dense frontal bone superiorly and supraorbital rim inferiorly; these are the thickest and strongest boundaries. In between these buttresses, the anterior wall becomes more attenuated and somewhat weaker across the face of the sinus. The thinner cortical posterior wall of the sinus is adjacent to the intracranial cavity and skull base. In the midline, the posterior wall of the frontal bone is adjacent to the crista galli, which rises superiorly from the cribriform plate; laterally, the cribriform plate transitions to the fovea ethmoidalis of the anterior skull base (Fig. 19-3). On the deep surface, the dura is dense and tightly adherent and thins along the midline of the anterior skull base due to multiple neurovascular foramina.15 Notably, after complete pneumatization of the sinuses, the final position of the cribriform plate is variable and often becomes situated in a position well inferior to the neighboring ethmoid air cells.
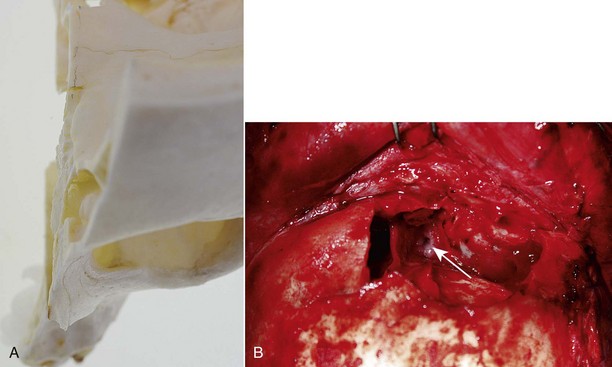
The posteromedial portion of the sinus floor contains an ostium that is the beginning of the nasofrontal outflow tract (Fig. 19-4). The lateral aspect of the floor is confluent with the medial portion of the orbital roof. Anteriorly and inferiorly is the nasoethmoidal complex, which has a significant likelihood of concurrent injury. This may manifest with telecanthus, loss of nasal bridge support, and medial orbital wall fractures. The relevance of these injuries in the context of frontal sinus trauma is their relationship to nasofrontal outflow tract obstruction as well as the position of the cribriform plate and potential for intracranial violation.
The arterial supply to the frontal sinus region is from the anterior ethmoid artery and the branches of the sphenopalatine artery via the middle meatus.2 The supraorbital, anterior superficial temporal, anterior cerebral, and middle meningeal arteries all supply the frontal bone.16 Venous drainage is transosseous into the subcutaneous, orbital, and intracranial veins. The diploic veins of Breschet are associated with foramina in the frontal bone significant for deeply invaginating sinus mucosa, which can be a source of mucocele formation if incompletely removed during obliteration or cranialization procedures. Also, they allow for direct vascular connections between the mucosal and dural venous systems.3,12,17,18 This pattern of venous drainage has clinical implications for the development and management of intracranial abscess associated with frontal sinusitis and infection. The frontal sinus is innervated by nerves that follow arterioles including the lateral posterior superior nasal branches of V2, as well as the anterior ethmoid nerve branching from V1.19
Normal sinus function is maintained by pseudostratified, columnar, ciliated respiratory epithelium covered by a layer of mucin. The cilia beat at the rate of about 10 to 15/second, with mucociliary clearance from the frontal sinus into the middle meatus of the nose.10,20 Interestingly, the frontal sinus is the only sinus in which there is some retrograde flow of mucus, with movement superiorly along the medial wall, laterally along the superior aspect, and then back to the ostium along the inferior aspects of the sinus. The rate is slowest at the roof of the sinus and the fastest around the nasofrontal duct.13,21
Pathophysiology
The location of the frontal sinus allows it to serve a protective role to the brain, in addition to providing normal sinus function. Although the presence of a frontal sinus has been confirmed to increase the likelihood of frontal bone fracture, it acts as a shock-absorbing barrier to the intracranial contents.22 The frontal bone at the anterior sinus wall is able to withstand direct trauma up to 990 kg of force.23,24 Conceptually, this force can be attained by an unrestrained passenger suffering a motor vehicle collision at 30 mph.25 As the frontal bone’s protective capacity is exceeded, concomitant intra- and extracranial injuries should be anticipated. The surrounding bones of the anterior skull base, orbits, and nasoethmoid complex are significantly weaker, leading to their potential for associated fracture. The posterior wall and anterior cranial base are particularly concerning because of the potential for dural tears, leading to communication between the intracranial compartment and sinus environment, with the possibility of meningitis or brain abscess.
History of Treatment
Complications from frontal sinus trauma and sinusitis in the age before antibiotics could be devastatingly morbid and frequently lethal. The history of surgical interventions was largely based on the treatment of chronic suppuration and sinusitis. The earliest reported operation on the frontal sinus was by Viega in 1586 for treatment of a frontal osteoma.26 As early as 1870, an attempt at surgically treating a frontal pyocele by external and intranasal approaches was published by Wells.5,27 Soon after, reports of treatment for infection of the frontal sinus by puncturing the frontal recess to improve drainage or pack the sinus followed, but were not embraced secondary to the risk of inadvertent entry into the intracranial space.
Jacobs credits Ogston in 1884 with the first substantial description of an external approach to the frontal sinus to establish drainage, outflow tract dilation, and intranasal drain placement.5,28 Reidel, in 1898, reported on a radical exenteration of the sinus walls and supraorbital bar, followed by removal of all sinus mucosa and leaving the overlying soft tissues to retract into the defect against the posterior wall.26 The hope was to reduce the potential for mucocele, mucopyocele, meningitis, or brain abscess, but at the cost of profound disfigurement.
In 1903, Killian presented a more conservative variation of Reidel’s procedure that maintained the supraorbital bar limiting the ablation to the anterior table and frontal sinus mucosa while using postoperative stents in the outflow tract.5,29 Despite the limited improvement in cosmesis, morbidity and mortality remained high secondary to persistent disease, most likely from postoperative closure of the outflow tract.30 This too was quickly discarded as a viable treatment option.31
By 1921, Lynch had introduced a promising procedure whereby a medial periorbital incision was used for access to the floor of the frontal sinus and anterior ethmoid air cells, with the aim of extirpating the mucosal lining and opening the nasofrontal drainage system, again using stents to maintain the sinus drainage.32 Despite the appeal of a more limited operation, results with this technique were also disappointing because complete removal of the mucosal lining was difficult and restenosis of the nasofrontal outflow tract was common.33
Fractures of the frontal sinus in the first half of the twentieth century were generally approached nonoperatively and most healed uneventfully.34 Even so, compound fractures were expected to have high mortality, approaching 50% if untreated when associated with pneumocephalus.35 Treatment was still required in select circumstances and Jacobs has stated thatt because of “continued reports of at least a 30% failure rate with the Lynch frontoethmoidectomy, as well as the associated difficulty in visualizing the distal portions of the frontal sinus, the osteoplastic school began to gain acceptance.”5
Beginning in 1934, Bergara and Itoiz developed the osteoplastic flap approach, in which the anterior frontal sinus wall was removed but maintained on an inferior pedicle of pericranium to allow replacement of the bone flap.36,37 This procedure allowed improved access to the damaged sinus so that removal of the mucosal lining could be accomplished more thoroughly, all while preserving the anterior table for a more natural-appearing reconstruction. The use of adipose tissue for obliteration of the frontal sinus can be traced back to Marx in 1910,26 but Bergara and Itoiz36,37 and later Montgomery scientifically examined and popularized the technique, publishing a series of papers emphasizing the importance of nasofrontal drainage function and frontal sinus obliteration with autologous fat to prevent inflammatory complications.38,39
Once considered the gold standard for chronic frontal sinusitis, the osteoplastic flap has since fallen out of favor because of its associated complications—cerebrospinal fluid (CSF) leak, frontal bossing, supraorbital neuralgia, chronic sepsis, mucocele formation, and osteitis.40–42 With the development of modern craniofacial techniques, it became clear that wide subperiosteal undermining, most often through a coronal scalp incision along with primary bone graft reconstruction, were viable strategies in the craniomaxillofacial region. Subsequently, this led to further improvements in surgical access and more aggressive attempts at primary reconstruction using these principles. Impressive results, with low morbidity after mucosal exenteration and fat graft obliteration of sinuses with injured nasofrontal ducts according to these methods, have since been reported.6,43
Removal of the posterior wall of the frontal sinus in cases of severe comminution or pneumocephalus had been described earlier,34,35 but cranialization of the frontal sinus in its contemporary form can be attributed to Donald and Bernstein.44 Encouraged by the work of Nadell and Kline performing primary reconstruction of depressed skull fractures in penetrating cranial injury with low rates of infection,45 they described a procedure in which the posterior frontal sinus wall was removed, all sinus mucosa was eliminated, and the intracranial contents were isolated from the nose by obstructing the nasofrontal outflow tract. Importantly, they advanced the idea that one could reconstruct the anterior table, even in the setting of contaminated injury after disinfection. This provided improved cosmetic and functional results in the group of patients with complex frontobasilar injury. Since that time, treatment has largely focused on variations of the techniques of cranialization, obliteration, anterior table reconstruction, and management of the nasofrontal outflow tract.
Overview of Clinical Decision Making
Goals of Treatment
A review of the literature provides little definite direction regarding the management of patients with frontal sinus injuries. Most studies are retrospective reports and series, with biased samples and limited follow-up. Treatment outcomes are poorly documented, as are the effects of operative versus nonoperative management. Importantly, Chuang and Dodson have suggested that we do not know how often bad outcomes occur in the untreated injury and we have no information to support that treatment decreases the risk for an adverse outcome.46 Treatment recommendations are therefore based on theoretical outcome predictions according to knowledge of frontal sinus pathophysiology and local intracranial vulnerability, which likely encourages overtreatment with the hope of minimizing complications. Further work will hopefully bring additional clarity to the proper use of current techniques.
Epidemiology
Since frontal sinus development is not appreciable in early childhood and gradually enlarges throughout the teen years, fractures are rare in children and uncommon in adolescents.47,48 For those treating adults, frontal sinus trauma remains infrequent being reported in 5% to 15% of all facial fractures from major trauma centers and presumably represents a much smaller proportion at community centers.13,34,49–57 In the contemporary civilian literature, frontal sinus injuries are frequently associated with blunt trauma secondary to motor vehicle accidents (MVAs), causing demographics to skew toward major trauma statistics.53,55,58 Although young and old are reported, the average age is consistently in the fourth decade and the overwhelming majority of injured are male (Fig. 19-5).50,51,59,60 Associated maxillofacial fractures may be present in over 70% of patients.58,61 Regarding the sinus itself, most fractures involve the anterior table alone (43% to 61%) or the anterior and posterior tables (19% to 51%). Frontal recess or frontonasal floor involvement with possible disruption of the drainage system occurs less commonly (2.5% to 21%). Isolated injuries of the posterior table are rare (0.6% to 6%).57,62,63
Fracture Classification
There is no universally accepted classification of frontal sinus injuries and specific numbering systems are largely academic. From a clinical perspective, distinction is commonly made between lateral and central injuries.52,64 Injuries of the lateral fronto-orbital skeleton frequently involve the supraorbital margin and lateral orbital wall, as well as occasionally involving the temporal or parietal skull. Care is focused on assuring dural and cranial vault integrity and the reconstruction of orbital volume, with limited regard for sinus involvement.
Central injuries are directly related to the frontal sinus and further distinction should be made based on the integrity of the naso-orbital-ethmoid (NOE) complex12 and skull base.65 Classification here is divided into specific functional and anatomic units, including the anterior table, posterior table and skull base, dural competency (cerebrospinal fluid leakage), NOE complex and nasofrontal outflow tract integrity. With the ready availability of high-resolution CT imaging, diagnosis and distinction of these injuries are realistic.
Numerous treatment algorithms can be found throughout the literature.15,58,61,66 Similar to classic facial trauma treatment principles, an inside-out approach is advocated that emphasizes the importance of frontobasilar integrity and seeks functional sinus preservation, when possible.
Diagnosis of Frontal Sinus Injuries
History and Physical Examination
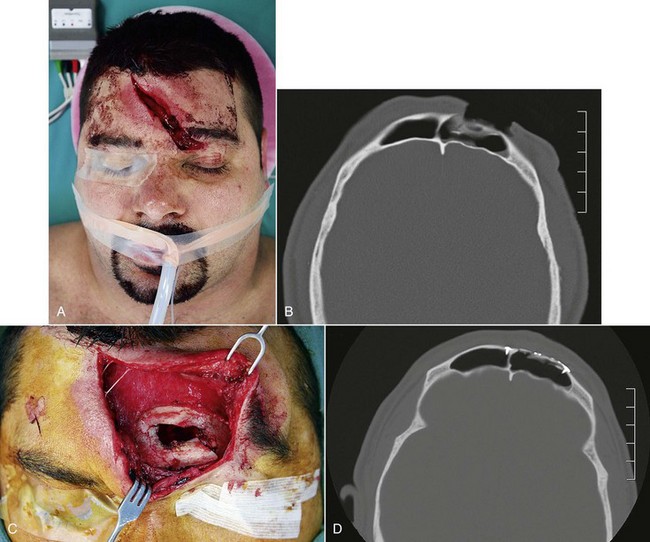
Frontal sinus fractures are commonly caused by high-velocity events and are frequently associated with other systemic or craniomaxillofacial injuries.51 Over 50% of patients diagnosed with frontal sinus fractures will suffer neurologic injury, with as many as 25% demonstrating associated ophthalmologic injury.12 Subdural or epidural hematomas requiring acute neurosurgical intervention may be found in as many as 8% to 10%.67 Rodriguez et al have found cervical spine injuries in 7% to 14% of his series, depending on the severity of the injury.58 Prior to arrival at the hospital, patients are regularly intubated, sedated, and medically paralyzed. Initial evaluation often occurs in the emergency department or intensive care unit and the most of the history is often taken from first responder records and other medical staff, depending on the condition of the patient and availability of family members or historians. Even in the setting of an apparently isolated frontal sinus injury, one needs to maintain a high degree of suspicion for other craniomaxillofacial and anatomically remote injuries. A comprehensive and systematic trauma examination is always indicated and reviewed elsewhere in this text.
The nasal cavity should be gently cleaned and inspected for septal or mucosal injuries. The presence of rhinorrhea or otorrhea is not uncommon in the setting of complex midfacial trauma and may be related to CSF leakage. Identification of a CSF leak is important, although at times difficult, because this finding specifically alters patient and injury management. In a series by Bell and Chen, rhinorrhea was present in 26% of frontobasilar fractures, but CSF leakage in only 4.6%.51 If cooperative, patients may be asked to lean forward and perform the Valsalva maneuver to increase CSF flow or may be asked about posterior nasal drainage. Use of the ring or halo test can be suggestive of the presence of CSF. Suspected fluid is placed on gauze or filter paper and an inner ring of blood may be seen, surrounded by an outer ring of clear CSF. Assessing the chemical composition of the fluid may also provide additional diagnostic support because CSF has a higher glucose concentration and lower chloride concentration compared with serum. Importantly, the presence of the beta-2 transferrin isoenzyme is most diagnostic.68 However, collected fluid requires electrophoresis for separation of the proteins and the Western blot technique to detect the beta-2 transferrin isoenzyme, which may take up to 4 days to process in the laboratory, resulting in a delay in diagnosis.69
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses