CHAPTER 18 Agents Used in General Anesthesia and Sedation
General anesthesia can be induced by many compounds of diverse chemical structure, including inorganic compounds, halogenated hydrocarbons, simple alcohols, aromatic agents, steroids, and other drugs that affect the central nervous system (CNS). General anesthetics are available as gases, volatile liquids, and solutions suitable for parenteral injection. When administered in lower doses, many of these same drugs may cause clinically useful sedation. This chapter describes the pharmacologic features of drugs used in general anesthesia and various levels of sedation. The application of these agents in dentistry, in particular for sedation, is reviewed in Chapter 48.
INHALATION AGENTS
Gases and volatile liquids are the oldest known anesthetic agents and have been the most widely used. Today, the only commonly used gas is nitrous oxide. Although general anesthetic agents administered by inhalation are often divided into gases and volatile liquids, there are few differences between these two classes of substances other than boiling point (Table 18-1) and solubility in various tissues (see Table 17-2). Regarding boiling point, which determines the vapor pressure of the gaseous phase, liquids need vaporizers, which produce and maintain an adequate amount of anesthetic in the inspired air. Because tissue solubility (i.e., solubility in brain membranes) is normally greater with the volatile liquids than gases, a smaller concentration of volatile agent is required in the inspired air to produce general anesthesia. An ideal inhalation anesthetic should possess numerous characteristics as outlined in Box 18-1.44
Nitrous Oxide
Nitrous oxide is arguably the oldest general anesthetic agent (see Chapter 17 for a review of the discovery of anesthesia) and the only gaseous anesthetic currently in use. Nitrous oxide is also the only inorganic substance used clinically as an anesthetic. Several features unique to nitrous oxide among available agents include a minimum alveolar concentration (MAC) greater than 100%, strong analgesic properties in subanesthetic concentrations, and minimal relaxation of skeletal muscle.
Physical and chemical properties
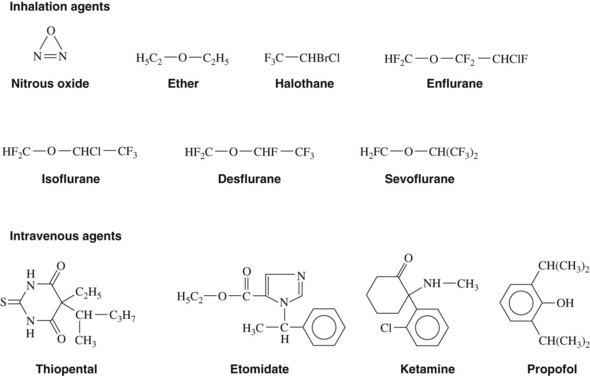
Nitrous oxide is a colorless, nonirritating gas with a pleasant, mild odor and taste. The structural formula is shown in Figure 18-1. Its blood/gas partition coefficient of 0.47 means that it is poorly soluble in blood. It is nonflammable but can support combustion in the absence of oxygen. It is available in pressurized steel cylinders as a liquid in equilibrium with its gas phase. As the nitrous oxide gas is delivered from the cylinder, liquid nitrous oxide spontaneously vaporizes to replace the lost gas phase. Cylinder pressure is maintained unaltered by this process until all the liquid has vaporized, at which point approximately four fifths of the contents have been released. This vaporization process requires heat, which is provided from the cylinder and the air around it, causing the tank to become cold.
Anesthetic properties
In dentistry, nitrous oxide is usually administered in subanesthetic concentrations of 20% to 50% to provide mild-moderate sedation and analgesia. Concentrations above this range may impair the patient’s ability to maintain consciousness and lead to a greater incidence of adverse effects, such as nausea or dysphoria. At a 40% concentration, there is good hard and soft tissue analgesia. Awareness of sensory input is reduced, with the exception that sounds may seem louder and qualitatively different.94
When nitrous oxide is used with a more potent agent, it is possible to reduce the concentration of the other drug and still achieve a more rapid induction and a shorter recovery period. This phenomenon is a reflection of the fact that the MAC of the rapidly acting nitrous oxide is additive with that of other, slower acting inhalation anesthetics. The addition of 70% nitrous oxide, which is approximately 0.6 MAC, reduces the MAC of halothane from 0.75% to 0.29% and the MAC of isoflurane from 1.15% to 0.5%, each approximately a 60% reduction. In addition, the concentration and second gas effects described in Chapter 17 can help hasten the onset of anesthesia.
Cardiovascular effects
In contrast to the volatile anesthetics in current use, nitrous oxide does not usually produce any clinically significant cardiovascular effects. It has a weak, dose-dependent myocardial depressant effect and a mild sympathomimetic effect.46 These opposing influences tend to cancel each other, leading to minimal to no change in cardiac output. Patients at increased risk of the cardiac depressant effects of nitrous oxide include patients with chronic hypertension, left ventricular failure, and advanced atherosclerotic disease.
Respiratory effects
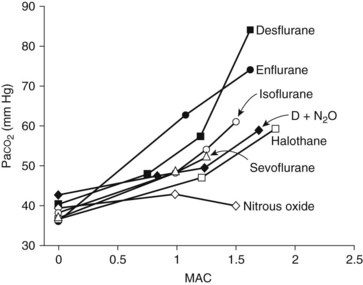
Nitrous oxide is not a strong respiratory depressant (Figure 18-2), but it decreases tidal volume and increases respiratory rate. Even so, there is likely to be less respiratory depression than would be caused by an equal depth of anesthesia induced by a single potent anesthetic drug. Although nitrous oxide has little effect on respiration in normal individuals, whose ventilation is regulated by the arterial carbon dioxide tension (Paco2), patients with severe chronic obstructive pulmonary disease whose ventilatory drive depends on the arterial oxygen tension may become severely hypoxic on exposure to even sedative concentrations of anesthetic.101 Even if hypoxemia is prevented by the high concentration of oxygen that is being coadministered (which by itself blunts the hypoxic drive for respiration), hypoventilation and respiratory acidosis are likely outcomes.
Adverse effects
When used for sedation, nitrous oxide usually provides a feeling of relaxation, along with the possible symptoms of body warmth, tingling of the hands and feet, circumoral numbness, auditory effects, and euphoria. As the dose increases, the patient is more likely to develop adverse symptoms such as dysphoria and nausea.27,28 Some patients may develop acute tolerance to these effects.75
For general anesthesia, high concentrations are used, and because its solubility in blood greatly exceeds that of nitrogen, nitrous oxide increases the volume of any enclosed air pocket in the body. There are several situations in which this property can be problematic: with a pneumothorax or lung bullae, injection of air into the ventricles during a pneumoencephalogram, an obstructed bowel, a blocked eustachian tube (with potential damage to the tympanic membrane), or after eye surgery that uses intraocular gases. With respect to vitreoretinal surgery, such as the surgical repair of retinal detachments and macular holes, perfluoropropane or sulfur hexafluoride is introduced within the eye to act as a tamponading agent. These gases may persist in the eye for up to 3 months. Administration of general anesthesia during this interval has led to case reports of irreversible loss of vision.9,35,96 These case reports suggest that nitrous oxide should be avoided in patients who have had vitreoretinal surgery with intraocular gas infusion in the past 3 months.
Nitrous oxide is not acutely toxic, but it can affect DNA synthesis by inducing changes in folate and amino acid metabolism. Its administration leads to an increase in homocysteine and 5-methyltetrahydrofolate.33 Nitrous oxide oxidizes the cobalt atom in vitamin B12, which renders inactive the vitamin B12–dependent enzyme methionine synthase. Methionine synthase is required to form the essential amino acid methionine (from homocysteine) and to transform 5-methyltetrahydrofolate into an active form for subsequent reactions. The enzyme is quickly inactivated in vivo by brief exposures to nitrous oxide.25,56 This inactivation increases with the nitrous oxide concentration and duration of exposure, is permanent, and requires synthesis of new enzyme for restoration of normal metabolism.55,80 Methionine deficiency is believed to be associated with degenerative nervous system changes. It has been suggested that preoperative administration of methionine may counteract some of the adverse effects of nitrous oxide on the hematologic and nervous systems,17 and methionine has been used in the treatment of nitrous oxide–induced neuropathy.91
Continuous inhalation of nitrous oxide can result in altered hematopoiesis because of the suppression of DNA synthesis. Patients exposed to 50% nitrous oxide for 6 hours may begin to show evidence of impaired thymidylate metabolism; hematopoietic changes suggestive of pernicious anemia occur after 24 hours of continuous inhalation.1 Intermittent exposures have a cumulative effect if spaced more frequently than once every 3 to 4 days.71 These findings have limited the use of nitrous oxide as an analgesic agent for extended use and for procedures that must be repeated often, such as debridement of burned skin.
The inhibition of methionine synthesis by nitrous oxide has been associated with an increased risk of myocardial ischemia in patients undergoing vascular surgery.4 Patients at special risk include patients with genetic mutations that cause a deficiency in 5,10-methylenetetrahydrofolate reductase activity.70 This enzyme generates the 5-methyltetrahydrofolate required for methionine synthesis; its deficiency potentiates the pathway block caused by nitrous oxide. Pretreatment with B vitamin supplements for 1 week before anesthesia can prevent the hyperhomocysteinemia believed to cause these adverse effects.
Similar to other mood-altering drugs, nitrous oxide may be abused by individuals with access to the drug, including members of the dental profession. This abuse is associated with myeloneuropathic changes indicative of a pernicious anemia–like syndrome: numbness and paresthesia, muscular weakness and incoordination, altered spinal reflexes, impotence, and shooting sensations on flexion of the neck (Lhermitte’s sign).62
Nitrous oxide has been shown to inhibit the release of luteinizing hormone–releasing hormone by the hypothalamus, which theoretically may impair fertility.59,60 Potential reproductive toxicity has also been proposed to be caused by the sympathomimetic effects of nitrous oxide leading to vasoconstriction and diminished uterine blood flow.36,68 Clinical use in pregnant women carries no apparent increased risk to the fetus, however, over other acceptable forms of pain control.23,67 Long-term exposure has been strongly implicated in other reproductive abnormalities, such as spontaneous abortion19 and impaired fertility,79 but these effects have not been substantiated by controlled prospective studies.
The possibility that long-term exposure to trace concentrations of nitrous oxide may be a health hazard to dental office and operating room personnel is discussed in Chapter 17.19 An early report of inhaled concentrations of as little as 50 ppm over a 2-hour span causing impairment in audiovisual performance tasks12 has not been reproduced.21,89 Nevertheless, this finding prompted the National Institute for Occupational Safety and Health to recommend 25 ppm as a maximum permissible time-weighted exposure limit per anesthetic administration for all health care workers. This level may not be achievable with some existing scavenging systems,100 so other measures (e.g., using rubber dam, using high-velocity suction, limiting the patient’s talking) must be used to minimize the gas escaping into the room. As discussed in Chapter 17, the issue of controlling waste anesthetic gas in the workplace continues to evolve.
Therapeutic uses
Nitrous oxide is a widely used inhalation anesthetic and continues to play a major role in the delivery of medical and dental anesthesia. It is valuable in reducing the concentration of volatile anesthetics during inhalation anesthesia and as a component of “balanced anesthesia.”* Historically, nitrous oxide was first used for dental surgery, but with the advent of local anesthetics, it was replaced as the drug of choice for providing pain control sufficient for most dental procedures. Since the late 1950s, there has been an upsurge in the use of nitrous oxide, not to provide dental anesthesia, but to provide relief from anxiety in the form of minimal-moderate sedation. In this role, it is often the agent of first choice. Its therapeutic application in dentistry is described in Chapter 48. Conversely, its use for general anesthesia in medicine is declining because of the increasing reliance on intravenous anesthesia coupled with concerns about occupational exposure to the gas. In these settings the potential for nitrous oxide to increase homocysteine concentrations and the risk of postoperative vascular thrombosis, myocardial ischemia, and stroke become significant.
Ether
Ether (diethyl ether) was the most widely used volatile anesthetic in the century that followed the first successful demonstration of general anesthesia in 1846. As described in Chapter 17, the sequential effects of ether inhalation were the basis for Guedel’s stages of anesthesia. Ether has been superseded by newer inhalation agents and is rarely used as a general anesthetic in North America. A brief description is included because of its historical importance.
Halothane
Physical and chemical properties
Halothane is a halogenated hydrocarbon; it is nonflammable, has a characteristic sweet odor, and is available in brown glass bottles with thymol added to maintain chemical stability. Its physical and solubility properties are summarized in Tables 17-2 and 18-1.
Anesthetic properties
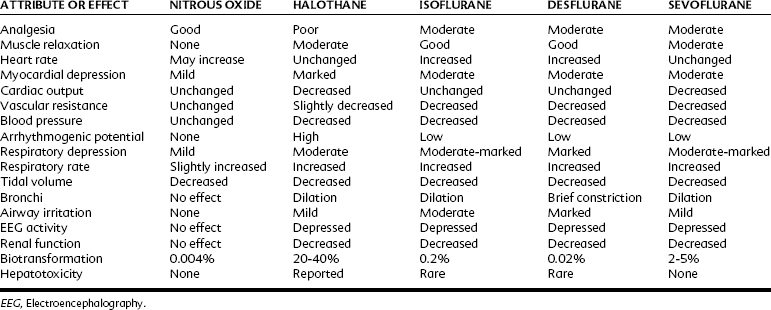
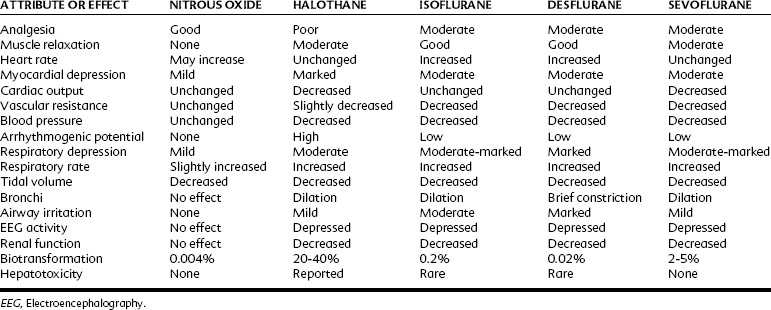
Table 18-2 compares the pharmacologic properties of halothane with other inhalation anesthetics. With a MAC of 0.75%, halothane is a potent general anesthetic that can be administered with excess amounts of oxygen. With its blood/gas partition coefficient of 2.5, the induction time of halothane is faster than that of older drugs such as ether, but slower than that of nitrous oxide and the newer volatile agents currently in use. Halothane has poor analgesic properties; at surgical anesthetic levels, an unconscious patient may respond to a noxious stimulus with increased motor activity and alteration of autonomic parameters. For this reason, halothane is most often used with nitrous oxide or an opioid analgesic or both. Because halothane produces incomplete muscle relaxation, it is also often combined with neuromuscular blocking agents.
Cardiovascular effects
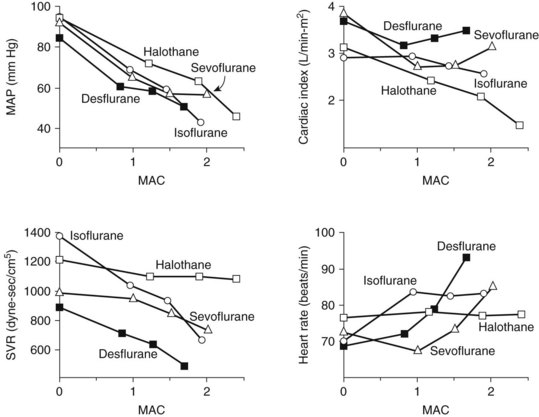
Halothane decreases the mean arterial blood pressure, primarily as a result of decreased cardiac output, and at 1 MAC it decreases by 25% (Figure 18-3). The decrease in cardiac output is greater than that found with equipotent amounts of isoflurane. Halothane, similar to other volatile general anesthetics, has a direct, significant, and dose-dependent depressant effect on myocardial contractility and, to a lesser degree, on vascular smooth muscle.81 The negative inotropic effect is attributed to a decrease in the influx of Ca++ through the slow channels of the sarcolemma, a decrease in Ca++ accumulation in the sarcoplasmic reticulum, and a decrease in Ca++ sensitivity of contractile proteins.
Halothane exerts a direct negative chronotropic effect at the sinoatrial node as a result of reduced cardiac sympathetic activity and vagal predominance. This depression leads to a slowing of the heart rate and possible junctional rhythms. Halothane also depresses the baroreceptor reflex and suppresses the expected increase in heart rate caused by hypotension.5 Halothane is a vasodilator; the peripheral systemic vascular resistance may be decreased, especially in patients with high sympathetic tone (e.g., patients with congestive heart disease or hypertension).
There is no stimulation of sympathoadrenal discharge with halothane and no increase in plasma catecholamines. Halothane sensitizes the myocardium to catecholamines, however, which can predispose to cardiac dysrhythmias,7 and this effect may be potentiated further by thiopental or hypercarbia. Dysrhythmias can occur as a result of catecholamines released endogenously in response to an elevated Paco2 or surgical stress, or after the injection of pressor agents given to augment blood pressure. Of direct relevance to dentistry is the use of epinephrine as the vasoconstrictor in local anesthetics and gingival retraction cords. For submucosal administration, it is recommended to limit exogenous epinephrine administration to 1 µg/kg if halothane is used with thiopental and 2 µg/kg if used alone.18 Avoidance of hypoxia, hypercarbia, and electrolyte abnormalities also reduces the likelihood of this adverse event.
Respiratory effects
Halothane induces dose-dependent respiratory depression. At light anesthetic levels, breathing becomes shallow and rapid, and the Paco2 is maintained at a concentration 25% higher than normal (see Figure 18-2). Tidal volume decreases. As with all inhalation anesthetics, the ventilatory response to carbon dioxide is diminished, and controlled ventilation is frequently necessary in deeper planes of anesthesia. Halothane virtually eliminates the respiratory stimulant effect of hypoxia at concentrations ≥0.1 MAC. Halothane is an effective bronchodilator, which is beneficial in the asthmatic patient.
Metabolism
A significant portion (≥20%) of the administered halothane is biotransformed in the liver, primarily by oxidation by the cytochrome P450 microsomal oxidase system.14,24 Reduction accounts for 2% of the metabolism. In contrast to other inhalation anesthetics, hepatic metabolism is an important contributor to the elimination of halothane. The metabolites include trifluoroacetic acid, which may be responsible for toxic effects in the liver (as described later), and Cl− and Br−.
Adverse effects
Halothane has been associated with delayed hepatotoxicity, which may manifest as one of two syndromes.29,32 The first is a mild, self-limited form of liver dysfunction that may occur after an initial exposure and has an incidence approximating 20%. This disturbance, usually recognized by an increase of liver enzymes in the plasma, may result from a direct effect of the drug or its metabolites. It may be exacerbated by liver hypoxia because it is strongly associated with impaired hepatocyte oxygenation because of preexisting liver disease, hypoxemia, or decreased hepatic blood flow.
The second syndrome, known as halothane hepatitis, is characterized by the development of massive hepatic failure with a high mortality rate.10 This latter syndrome has an incidence approximating 1 : 10,000 in adults (1 : 100,000 in children) and is associated with repeated exposure to halothane. The clinical features of halothane hepatitis include gastrointestinal upset, jaundice, fever, rash, eosinophilia, and serum autoantibodies.5,77 This more fulminant form is caused by an immunologic mechanism. The oxidative metabolism of halothane leads to a reactive trifluoroacetyl halide metabolite. This metabolite induces antigenic changes to hepatic microsomal proteins, producing neoantigens and subsequent autoantibodies. Because of this syndrome, halothane is generally contraindicated in adults, especially individuals who have previously been exposed to halothane, or in any patient regardless of age who has shown signs of liver toxicity on previous exposure to halothane or related anesthetics.32,78 Halothane is also contraindicated for any abdominal surgery likely to decrease the alveolar ventilation or liver blood flow.
Malignant hyperthermia is a rare adverse effect of general anesthesia involving halothane, other volatile anesthetic agents, and the neuromuscular blocking drug succinylcholine. In the United States, the incidence of malignant hyperthermia is 1 : 50,000 in adults and 1 : 15,000 in children. It is a genetic disorder of multifactorial etiology. Most cases are associated with mutations in the ryanodine receptor (type 1), which forms a Ca++ channel in the sarcoplasmic reticulum and is involved in Ca++-induced Ca++ release. Malignant hyperthermia can be associated with the following disorders: central core disease; Duchenne’s muscular dystrophy; King-Denborough syndrome; other myopathies; and musculoskeletal congenital defects, such as cleft palate, scoliosis, clubfoot, ptosis, strabismus, cryptorchidism, and congenital hernias.11,92
An acute crisis of malignant hyperthermia is a hypercatabolic reaction that often manifests initially as masseter or generalized muscle rigidity; other early signs include elevation of oxygen use and carbon dioxide production, tachypnea, and tachycardia. Cardiovascular instability, cardiac dysrhythmias, electrolyte disturbances, and elevation in temperature are other classic signs. The body temperature, often unaffected early in an acute attack, progressively increases to alarming and sometimes fatal levels. The elevated heat production, associated with increased Ca++ concentrations in the myoplasm and hypermetabolic activity of skeletal muscle, is responsible for the hyperthermia.41
Immediately on recognition, all triggering agents should be discontinued, and hyperventilation with 100% oxygen should be instituted. Dantrolene, an inhibitor of Ca++ transport (see Chapter 10), must be administered intravenously as soon as possible because this drug provides lifesaving, definitive treatment.40 Dantrolene should be administered as a bolus intravenously at a dose of 2 mg/kg to 3 mg/kg and then titrated in response to the patient’s clinical condition. If present, metabolic acidosis and any dysrhythmias or electrolyte disturbances should be treated. Cooling in the form of cold intravenous solutions, packing the patient in ice, and ice water lavage of body cavities should be performed to increase heat loss and reduce body temperature. Effective treatment rendered quickly after prompt recognition of malignant hyperthermia has reduced its mortality rate from 70% to less than 10%.
Isoflurane
Anesthetic properties
Cardiovascular effects
Similar to all volatile anesthetics, isoflurane produces a dose-dependent depression of myocardial contractility, but it is considerably less than that seen with halothane. Isoflurane also causes coronary vasodilation, mostly at the distal (resistance) arterioles.74 Although this effect may be beneficial for heart muscle, it was also proposed to cause “coronary steal” in patients with ischemic heart disease, a situation in which blood flow is redistributed from myocardial tissues supplied by atherosclerotic arteries to areas with healthy coronary vessels. Coronary steal develops only when the coronary perfusion pressure is decreased, is more likely to occur with excessive tachycardia, and is most probably not a special concern with isoflurane. Cardiac output is well maintained with isoflurane (see Figure 18-3), even though stroke volume is decreased, by virtue of an increase in the heart rate, showing isoflurane’s greater preservation of baroreceptor reflexes. Decreases in arterial blood pressure are similar to the decreases produced by halothane, however, because of the greater vasodilator effect of isoflurane. Isoflurane does not significantly sensitize the heart to dysrhythmias; the permissible injected dose of epinephrine during isoflurane anesthesia is three times that with halothane.
Respiratory effects
Respiratory depression is greater than that with halothane (see Figure 18-2) and manifests as a decreased ventilatory response to hypercapnia with a complete loss of sensitivity to hypoxia. Isoflurane increases respiratory rate only up to 1 MAC. Bronchodilation is similar to halothane.
Metabolism
In contrast to halothane, biotransformation of isoflurane is quite low (≤0.2%). This finding suggests that it is neither nephrotoxic nor hepatotoxic, a conclusion supported by observations that repeated and prolonged exposures to isoflurane have not caused hepatorenal injury in animals. It is biotransformed by the same enzymatic pathway as halothane. Although there are a few case reports of hepatic necrosis after isoflurane administration,15,39 it is currently believed that isoflurane is highly unlikely to be responsible for postoperative hepatotoxicity.77
Therapeutic uses
Isoflurane is a suitable drug whenever a potent inhalation anesthetic is to be administered except when a mask induction of anesthesia is contemplated. In pediatric patients, induction with isoflurane is more likely to elicit coughing, salivation, and laryngospasm69 than induction with halothane. These effects can be prevented by prior administration of an intravenous induction agent. Isoflurane has numerous advantages: it is chemically stable, nonflammable, and potent; induction is rapid, and muscle relaxation is adequate; and it is not dysrhythmogenic or toxic to the kidneys or liver. Isoflurane depresses the cardiovascular and respiratory systems. It is also contraindicated in patients with a history of malignant hyperthermia.
Desflurane
Physical and chemical properties
Desflurane is chemically very similar to isoflurane, with only a single substitution of fluorine for a chlorine atom (see Figure 18-1). Desflurane shows marked chemical stability, possibly because of the additional fluorine, which provides resistance to breakdown in soda lime and to biotransformation. The anesthetic has a high vapor pressure of 664 mm Hg at 20° C, becomes a gas (vapor pressure 760 mm Hg) at 23° C, and is not flammable at concentrations less than 17%. The low potency and high volatility of desflurane requires the use of a heated vaporizer to enable the delivery of this agent.13
Anesthetic properties
The low solubility of desflurane in blood results in rapid onset, recovery, and adjustment of anesthetic depth, similar to that found with nitrous oxide.31,102 A propensity to cause breath holding, coughing, and laryngospasm during mask induction precludes its routine use as a primary induction agent.
With a MAC of 6% (in middle-aged adults), desflurane is less potent than the other volatile agents. Its physiologic effects are similar, however, to those induced by isoflurane. The systemic vascular resistance, mean arterial blood pressure, and stroke volume are reduced, but the cardiac output is maintained by a progressive increase in heart rate.97 As shown in Figure 18-3, discernible increases in heart rate occur as the anesthetic concentration exceeds 1.25 MAC. Similar to isoflurane, desflurane theoretically may cause coronary steal in hypotensive cardiac patients.30 There is no significant sensitization of the myocardium to catecholamines. Desflurane causes a dose-related decrease in tidal volume and, despite an increase in the respiratory rate, a significant depression of minute ventilation. As with other halogenated ethers, respiratory depression is reduced if desflurane is used with nitrous oxide for anesthesia (see Figure 18-2).
Desflurane is contraindicated in patients susceptible to malignant hyperthermia because it can trigger the syndrome in the swine model and has been linked to malignant hyperthermia in the clinical setting. Because desflurane is notable for having minimal biotransformation, it has a very low likelihood for causing serious hepatotoxicity.54
Sevoflurane
First synthesized in the United States in 1968, sevoflurane became widely used in Japan in 1990 and available for clinical use in the United States in 1995. A pleasant odor, lack of airway irritation, and rapid onset of action make sevoflurane an attractive alternative to halothane for mask induction of anesthesia in pediatrics.63
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses