17
Health and safety at work
- Health and Safety at Work Act
- The Control of Substances Hazardous to Health
- Fire prevention
- Radiation protection
- Personal protection
- Clinical governance
- Risk assessment
Introduction
Dental staff must be aware of and deal appropriately with the health hazards which may occur in relation to dentistry. Responsibilities for health are governed by the Health and Safety at Work Act 1974. The Act seeks to protect all those at work (employers, employees and the self-employed) as well as members of the public who may be affected by the work activities of these people.
Under the Act, the employers’ statutory responsibilities are to:
- Prepare a written health and safety policy.
- Ensure effective risk assessments are carried out and recorded.
- Ensure the management systems in place provide effective monitoring and reporting.
- Ensure staff are all aware of and comply with health and safety policies and procedures.
- Review health and safety performance at least annually.
- Be aware of and investigate any significant health and safety failures or concerns.
The Health and Safety at Work Act encompasses:
- The provision and maintenance of safe equipment, appliances and systems of work.
- Safe handling and storage of substances or articles which may be potentially harmful to health.
- Maintaining a safe working environment without risks to health including the means of entrance and exit.
- Providing the required instruction, training and supervision to ensure health and safety.
The main responsibility for ensuring the health and safety of staff and for reducing risks to others affected by work activities (including members of the public) rests on employers (section 2 and 3 of the Health and Safety at Work Act 1974). However, failure of employees to discharge the responsibilities laid down by the Act may provide grounds for dismissal and can also lead to prosecution by the Health and Safety Executive.
Working environment
There are specific requirements relating to the environment within a practice or clinic. The temperature should reach at least 16 °C after 1 hour and thermometers must be provided in all rooms to check that this has been achieved.
Adequate ventilation is essential to minimise any risk of dangerous or irritant vapours from mercury, disinfectants, nitrous oxide and laboratory chemicals.
The Workplace (Health, Safety and Welfare) Regulations 1992 require enclosed workplaces to be ventilated with sufficient fresh or purified air. An open window will provide this in most cases, alternatively mechanical ventilation or air conditioning units could be considered. These should provide at least 5–8 litres per second of fresh (not recycled) air per occupant. The working environment normally has a relative humidity of between 40% and 70%.
There should be washing, toilet and changing facilities sufficient for all staff and patients. There must also be staff facilities to make hot drinks, heat food and also somewhere for them to eat, drink and relax.
Lighting should be sufficient to work safely without eyestrain and workstations should be arranged so that each task can be carried out safely and comfortably.
Room dimensions of the working environment must also be taken into account, allowing for enough free space for people to move around with ease.
Environmental noise: Noise pollution will depend on intensity and duration. As a simple guide it may be considered to be a problem if speech cannot be clearly heard by someone positioned two metres from the speaker.
Exposure to noise pollution can be minimised by:
- Ensuring that dental handpieces, ultrasonic scalers, aspiration equipment laboratory equipment and compressors are regularly serviced.
- Creating a low emissions working environment by using absorptive materials within the building to reduce reflected sound.
- Limiting time spent in noisy areas.
Materials
Control of Substances Hazardous to Health
The Control of Substances Hazardous to Health Regulations 2002 (CoSHH) provides a legal framework for controlling the exposure of people to hazardous substances, including microbiological hazards, arising from work activity. They impose a duty on employers to ensure safety measures are in place.
Hazardous substances can be liquids, solids, dust, powder or gases and are primarily categorised as irritant, corrosive, flammable, toxic or very toxic and will carry appropriate hazard warning symbols (Figure 17.1). The United Nations Globally Harmonised System of Classification and Labelling of Chemicals (GHS) replaces the European classification. The deadline for substance reclassification in the EU was 1st December 2010.
Figure 17.1 United Nations Globally Harmonised System of Classification and Labelling of Chemicals (GHS).
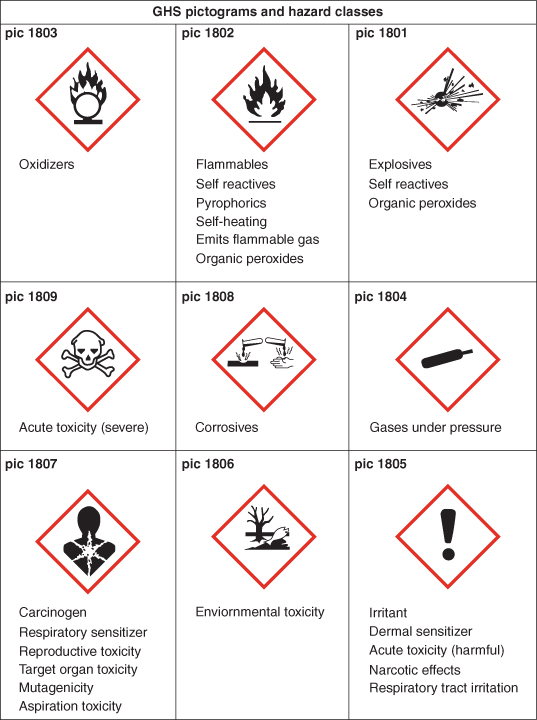
This information can be found on container labels or in product data. Many chemicals cause specific health effects by targeting various organs or parts of the body. The four main routes of entry are:
- Inhalation, via the bronchial system.
- Absorption, through the skin and eyes.
- Ingestion, through the mouth.
- Injection, via a puncture wound.
Information regarding microbiological hazards can be found in the Control of Cross Infection Guidelines and is dealt with fully in Chapter 8.
Under the CoSHH Regulations it is a requirement to identify and make an assessment of any substances hazardous to health in the practice or clinic. It is necessary to:
- Identify the substances in the practice that may be hazardous to health. These include such substances as mercury, acrylic monomer, volatile liquids, alginate dust and acid etch.
- Assess what the hazard is and who may be affected by its normal use.
- Decide whether the substance presents a hazard in normal use.
- Review the method and frequency of use, storage and disposal.
- Assess the overall risk that each substance poses within the practice.
- Assess the need for health and/or environmental monitoring.
- Compile and implement procedures to control/minimise the risks to health.
- Record the assessment.
- Provide training to ensure that all staff understand the CoSHH assessment and are aware of the appropriate procedures for the hazardous substances in the practice.
After completing a CoSHH assessment for each hazardous substance consideration should be given on how to minimise the risk to health.
Storage
All materials and chemicals should be stored in cupboards with isolated storage for inflammable substances and poisons. Mercury must be stored in a cool environment in sealed containers.
Medicines are required to be stored according to manufacturer’s instructions, have restricted and controlled access and stocks regularly checked for availability and shelf life.
Equipment
Electrical equipment
All electrical equipment must be maintained in a safe working order at all times. It is the responsibility of dentists or trained staff to undertake a regular (approximately every 6 months) visual inspection of cables, plugs and fuses and check that wires in the plugs are tightly attached to the correct terminals. Every 2–3 years all electrical equipment should be checked and tested by an appropriately trained person and records as evidence that this has been carried out must be kept.
Autoclaves and compressors
Before any autoclave or compressor is used, there is a requirement for a written examination procedure to be done, which includes the inspection intervals for defects in function or safety. All staff must be fully trained before operating autoclaves and the manufacturer’s recommended safety checks carried out before use.
The Health and Safety Executive has produced guidance to assist in the use of autoclaves, and the safeguards include:
- British Standards design.
- A safety valve to prevent over-pressurisation, a pressure indicator and drainage system.
- A safety door that cannot be pressurised unless the door is completely secured and the chamber sealed.
All autoclaves and compressors must be serviced and maintained at intervals specified by the manufacturer and records of these kept for audit and reference.
Computers/visual display units
Health and safety training is recommended to ensure employees can use all aspects of their workstation equipment safely and know how best to avoid health problems by, for example adjusting the chair, using a wrist pad and foot rest and positioning the desk and screen so that bright lights are not reflected onto the screen.
Computer work stations may be assessed for comfort with respect to operating position, monitor, mouse and keyboard. Lighting must be appropriate and anti-glare screens provided if considered appropriate. Eye tests can be arranged if necessary and corrective eyewear provided.
First aid
Every dental practice or clinic must have personnel trained to take charge of first-aid at all times. There is a requirement for the first-aid box to be easily located and clearly identified and it is a legal requirement for it to have a white cross on a green background.
The contents of the first-aid box should include sufficient assorted dressings, bandages and other suitable first-aid materials but not any medicaments or medicines. Provision of sterile water for eye irrigation must be provided where mains tap water is not available. It must be easily accessible to all members of staff and be regularly checked for out of date stock. A sign indicating the location of the first-aid box is not mandatory although desirable.
Cardiopulmonary resuscitation (CPR)
All members of the dental team are required to be trained and capable of undertaking CPR in the event of a medical emergency. Training should be given on a regular basis, preferably at least once a year, with ad hoc rehearsals. This is covered fully in Chapter 16.
Waste disposal
Dealing with sharps
Hazardous instruments, which may comprise of local anaesthetic needles, cartridges, scalpel blades, suture needles, dental burs and metal matrix bands are required to be placed in rigid puncture-proof UN sharps containers (to BS 7320) and filled only three quarters full to prevent personal injury (2010/13/EU Directorate). It is not acceptable practise to intentionally discharge syringes containing residual medicines in order to dispose of them in the sharps bin.
Disposal and use of extracted teeth
The Human Tissue Act 2004 covers the removal, storage and use of relevant material from the living; however, removal of teeth from the living is not included in the Act but once they have been extracted the use of these teeth and the storage of them is covered. The Act is supplemented by regulations, a number of which came into force on 1st September 2006.
Whilst consent may not be always required, any expressed wishes of a patient must be followed; so, for example, if a person expressly states that they do not want their teeth to be used for education or training this must be respected. Extracted teeth can be disposed of in clinical waste if they do not contain amalgam.
Waste segregation system
The management of waste is becoming increasingly complex as environmental issues become more prevalent. New guidelines have accordingly addressed this issue under the Hazardous Waste (Amendment) Regulations 2009. Waste must now be segregated in colour-coded bins and sacks. Household waste is disposed of in landfill and clinical waste is dispatched for either treatment or incineration, hazardous waste is stored in a leak-proof container. All categories must be stored in a suitable location prior to disposal. Hazardous waste includes mercury, dental amalgam, cytotoxic and cytostatic prescription only medicines, X-ray developer and fixer solutions. Partially used local anaesthetic cartridges are classified as clinical waste for treatment.
For further information refer to BDA guidelines or see the Department of Health website.
When clinical waste is collected, a transfer note must be completed and a copy retained for two years, by contrast for hazardous waste collection, a consignment note is required and a copy retained for 3 years.
Amalgam and mercury waste, including extracted teeth containing amalgam restorations, is classified as hazardous waste and cannot be incinerated due to the release of toxic mercury vapour. It should be stored separately in rigid airtight containers with a mercury suppressant and then collected by authorised contractors for recovery. It is the responsibility of the dental practice/clinic to ensure that only authorised persons collect clinical and hazardous waste. There is an obligation to check licences and registration certificates.
Amalgam separators
Strenuous efforts have been made within the EU since 1997 to reduce the amount of waste amalgam. Following the introduction of the Hazardous Waste Regulations 2005 all waste amalgam is now classified as ‘hazardous waste’ and discharge of it into the sewerage system is not allowed. All dental practices must have amalgam separators which comply with BS ISO EN 11143:2000 and ensure that amalgam is collected and disposed of in accordance with the regulations.
Mercury
This is one of the most hazardous substances used in dentistry and all staff must be aware of its potential hazards. Symptoms of chronic mercury poisoning (tiredness, fatigue and lethargy) are difficult to identify but deaths in dental practice from acute poisoning have been reported. There must be good general ventilation (air conditioning systems that recycle air are not recommended). Amalgamators are recommended to be placed on a shallow tray lined with aluminium foil or on a lipped plastic tray. A small funnel must be used when filling the mercury reservoir and a mercury spillage kit available for use if needed.
Using pre-dispensed amalgam capsules eliminates the risk of spillage but the amalgamator should be periodically checked for capsule leakage.
Aspirator tubing and bottles tend to collect mercury-rich amalgam when it is carved away. This type of equipment can thus become a hidden, but persistent source of mercury vapour. Traps should be emptied regularly and tubing cleaned daily with cleaning fluid recommended by the manufacturer.
To reduce the risk to health, all floor and work surfaces should cove slightly up the wall or cabinetry and joins must be sealed. Regular biological health monitoring for staff may be provided. Waste amalgam must be kept in a sealed container with a mercury suppressant.
If hands have been exposed to mercury, they should be washed immediately with liquid soap in a stream of cold water until no stain on the skin is seen. Disposable towels should be used for hand drying.
Mercury spillage
In the event of a spillage, the affected area must be confined to a minimum and protective gloves and mask worn. Increased ventilation is recommended by either opening a window or ensuring that air conditioning is working to maximum potential. The spread of the spill should be reduced as much as possible particularly avoiding mercury dropping onto the floor, however if this has occurred overshoes can additionally be worn.
Mercury spillage kits contain flowers of sulphur and calcium oxide. Using the brush or sponge from the kit, the globules of mercury are moved together to form one large pool and as much of this as possible should be removed using the kit brush and plastic scoop or sponge and placed in the waste container. A paste of flowers of sulphur, calcium oxide and water may be painted on and around the spillage and when dry wiped up using wet disposable tissues and transferred to the waste container. Merconspray is an alternative as this hinders the formation of methyl mercury. This has a shelf life of approximately 1 year.
The cap on the waste container should be replaced tightly, and the container stored in a well ventilated area away from heat sources. The work surface and /or floor can then be decontaminated using the procedure outlined below. The full waste container should be disposed of at a council approved site, where provision for toxic waste is made.
Decontamination procedure
Decontamination using the flowers of sulphur/calcium oxide mixture can be carried out routinely each month to reduce background exposure in the operating room, or after a spillage where the floor or work surfaces are affected. The mixture may be used on carpet if necessary and be followed by spot cleaning with a proprietary cleaner. A vacuum cleaner or aspirator should never be used to remove spilled mercury.
Detection of mercury vapour
If a serious spillage occurs, or there are other reasons for suspecting mercury contamination, equipment is available for determining the amount of atmospheric pollution. Any serious mercury spillage is recorded in the accident book and the Health and Safety Executive informed.
Accident book
This must be used to record all accidents to staff, patients or visitors which have occurred on the practice/clinic premises, regardless of how trivial they may seem. It should be readily available for inspection if required although because it contains personal information consideration should be given to confidentiality under the Data Protection Act.
Each entry records:
- Time, date and method of reporting.
- The nature of the accident.
- Where the accident took place.
- Who was involved.
- The action (if any) which was taken.
The accident book is useful for audit purposes and potentially improving the safety of procedures or the environment.
The Reporting of Injuries, Diseases and Dangerous Occurrences Regulations 1995 (RIDDOR/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


