8
Microbiology and infection prevention and control
- A requirement for a formal infection prevention and control policy
- An overview of potential transmissible pathogens of relevance to dentistry
- Practical guidance on the potential sources of infection in the dental environment and how to minimise cross-contamination and cross-infection risks
- Instrument decontamination, transportation and storage
- Effective waste management
- The dental chair unit as a vehicle for cross-contamination and cross-infection
Introduction
In the twenty-first century, should the prevention of transmission of infectious diseases be considered less important than dental treatment of patients? All dental healthcare professionals have a duty of care to their patients that includes the implementation of effective infection prevention and control (IPC) procedures designed to minimise the risk of transmission of pathogenic or potentially pathogenic microorganisms.
Dental healthcare personnel include all members of the dental team: dentists, dental hygienists, dental therapists, dental nurses, dental laboratory technicians (both working on-site in dental practices and hospitals and in commercial laboratories), students, contractors and other individuals not directly involved in patient care but potentially exposed to infectious agents (e.g. administrative, clerical, housekeeping and especially maintenance personnel). The purpose of IPC in the dental clinic setting is to reduce cross-contamination and to minimise risks associated with the transmission of infectious or potentially infectious microorgansisms. Preventative practices are used to minimise exposure to reservoirs of pathogenic microorganisms and environmental microorganisms, many of the latter of which are potentially pathogenic or behave as opportunistic pathogens, and to body fluids, particularly percutaneous (penetrated through the skin) exposures to blood. Such practices include:
- Careful handling of sharp instruments and equipment.
- The use of rubber dams and high-volume suction.
- Correct hand hygiene techniques.
- Appropriate use of protective barriers (e.g. gloves, masks, protective eyewear, gowns and surface barriers).
- Effective decontamination of contaminated surfaces, instruments and equipment.
Requirement for a formal infection prevention and control policy
All dental practices should have an effective IPC programme that includes the development and implementation of policies, procedures and practices to prevent work-related injuries and illnesses among dental healthcare personnel and support staff, as well as healthcare-associated infections among patients (Kohn et al., 2003). All dental team staff (including non-clinical staff) who may be subject to occupational exposure should receive regular and appropriate IPC training; they should be aware of the associated infection risks with all aspects of patient care, the proper maintenance, cleaning and disinfection of dental equipment and instruments and waste segregation. The effectiveness of the IPC programme should be evaluated by audit on an ongoing basis and reviewed periodically to ensure that the practices are effective and efficient and that the policy is implemented. These policies require the commitment and accountability of all staff concerned.
Microorganisms
It is beyond the scope of this chapter to provide a comprehensive overview of microorganisms and microbiology. There are numerous excellent textbooks and more general microbiology books available and we wish to encourage the interested reader to familiarise themselves with the more general aspects of microorganisms (Murray et al., 2009; Lamont, and Jenkinson, 2010). Microorganisms consist of bacteria (e.g. Escherichia coli and Staphylococcus aureus), fungi (e.g. Candida albicans and Aspergillus fumigatus), viruses (e.g. influenza virus and hepatitis B virus), protozoa, amoebae, mycoplasmas and rickettsiae. In relation to cross-infection in the dental clinic, the most significant microorganisms are bacteria, viruses and to a lesser extent fungi, the latter of which also include yeasts. Microorganisms are ubiquitous and occupy virtually every habitat, environment and niche in nature. Most of these organisms are totally harmless to humans and never cause us any problems. They are too busy just making a living (Figure 8.1).
Figure 8.1 The majority of microorganisms present in nature never cause infection. They are usually too busy just making a living.

Every individual comes into contact with numerous microorganisms every day, in the air, in water, in food and on both natural and artificial surfaces. Not surprisingly, a vast range of microorganisms is associated with the human body, the majority of which are usually harmless. In fact, many microorganisms that live on or in our bodies provide many beneficial effects and can be considered friendly microorganisms. These microorganisms are often referred to as commensals or the normal microbial flora and are present in enormous numbers on the skin, in the oral cavity and in the alimentary tract.
Microorganisms have always existed and evolved in close association with humans and we have developed complex and intricate specific (e.g. cell-mediated and humoral immunity) and non-specific (e.g. skin and epithelial barriers, and antimicrobial secretions such as saliva and tears) defence mechanisms to protect ourselves from becoming infected with microorganisms (Delves et al., 2011). Even when our bodies do become infected with microorganisms, our immune defence systems usually quickly eradicate the invaders. A relatively small number of microorganisms have developed specific mechanisms to exploit weaknesses in host defences that enable them to establish infections and cause disease. These organisms are known as pathogenic microorganisms or pathogens because they may cause disease in healthy individuals once they have gained access to the body. Examples include influenza virus, which causes flu; the bacterium Staph. aureus, which can cause skin infections and abscesses; and various strains of the bacterial species E. coli, which can cause diarrhoea. Many pathogenic microorganisms have evolved mechanisms to avoid or evade host defence systems and many in addition produce toxins (e.g. cholera toxin produced by the bacterial species Vibrio cholerae and diphtheria toxin produced by the bacterial species Corynebacterium diphtheriae) that, for example, can cause local tissue damage and/or more widespread systemic effects.
Another group of microorganisms can only cause infection and disease following a local or general defect(s) in host defence mechanisms. These microorganisms are sometimes referred to as opportunistic pathogens and frequently are naturally occurring environmental microorganisms found in air, water, soil, vegetable matter and on food. The normal commensal flora can behave as opportunistic pathogens following impairment in host defence (e.g. oral thrush caused by the oral yeast C. albicans following antibiotic treatment).
Recent advances in medical science and patient treatment have dramatically improved the quality of life and lifespan of countless individuals. Thus in recent decades there has been a significant increase in the proportion of individuals with compromised or impaired host defences, including human immunodeficiency virus (HIV)-infected and acquired immune deficiency syndrome (AIDS) patients, cancer patients and organ transplant recipients. These individuals are more susceptible to infection than normal healthy people and frequently become infected with opportunistic pathogens. Furthermore, many microorganisms, including human-derived and environmental organisms, have adapted to colonise a wide variety of medical devices used in patient treatment (e.g. urinary catheters, indwelling venous lines, oral prostheses, dental chair unit waterlines) and may give rise to serious and even life-threatening infections.
Infection prevention and control is a rational process that is implemented in the healthcare environment to minimise infection risks to patients and staff. The process, which has many aspects, centres on management of all aspects of the dental clinic environment, managing and minimising cross-contamination risks and thus minimising the potential for cross-infection. It is important to emphasise that cross-infection risks cannot be totally eliminated as we live in a world teeming with microorganisms. However, effective IPC programmes will dramatically reduce opportunities for cross-infection, thus providing a safe environment for patients and dental team staff and works best when all the aspects are implemented appropriately and meticulously (Smith et al., 2009).
Blood-borne viruses
The transmission of particular viral agents in blood and blood products has been identified as a serious and potentially fatal risk to healthcare workers, including dental healthcare professionals. These viruses include HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV). Transmission of blood-borne viruses in the dental healthcare setting is relatively uncommon but does occur (Shah et al., 2006; Redd et al., 2007). Exposure to virus-infected blood can result in transmission from patient to dental healthcare staff, from dental healthcare staff to patient and from patient to patient. The potential for transmission is greatest from patient to dental staff. The risk of exposure to blood-borne viruses by dental healthcare staff depends on the prevalence of the viruses in the patient population and the nature and frequency of contact with blood and saliva through percutaneous or sharps injury (e.g. needlestick injury). The risk of infection following exposure to a blood-borne virus depends on the inoculum size (i.e. the number of infectious virus particles to which an individual is exposed), the route of transmission (e.g. percutaneous injury with a needle contaminated with blood) and the susceptibility of the exposed healthcare worker.
Hepatitis B virus
HBV is transmitted by percutaneous and sharps injury or mucosal exposure to blood, saliva or other body fluids of individuals with either acute or chronic HBV infection. Blood contains the highest proportion of infectious HBV particles and is the most significant body fluid responsible for transmission in the healthcare setting. Infectious HBV particles have been shown to survive in dried blood at ambient temperature on environmental surfaces for up to a week (Bond et al., 1981). There is some evidence to suggest that some cases of HBV infection resulted from inoculation of HBV into cutaneous scratches, abrasions, other lesions, or on to mucosal surfaces. Fortunately, the incidence of occupational HBV infection among healthcare workers has decreased significantly over the last two decades because of vaccination and the implementation of effective IPC procedures. Safe and effective recombinant (i.e. genetically engineered) HBV subunit vaccines have been available for many years and all dental healthcare personnel should be vaccinated during training and before they are exposed to blood. These vaccines consist of viral protein purified from yeast cells and do not contain HBV particles.
Hepatitis C virus
HCV does not appear to be efficiently transmitted through occupational exposure to blood compared to HBV (Mitsui et al., 1992; Puro et al., 1995; Dement et al., 2004). Nonetheless, cases of transmission of HCV infection to healthcare workers including dental staff following exposure to HCV-infected blood have been reported following needle-stick injury or blood splashes (Shah et al., 2006). Currently there is no vaccine available for HCV.
Human immunodeficiency virus
In developed countries, the risk of HIV transmission in the dental clinical environment is very low. Prospective studies worldwide indicate that the average risk of HIV infection following a single percutaneous exposure to HIV-infected blood is 0.3%, and even lower following an exposure of mucous membranes in the mouth, nose or eye (Bell, 1997). Furthermore, the possibility of HIV transmission via saliva is remote (Navazesh et al., 2010). Currently there is no vaccine available for HIV but effective antiviral treatment regimens are available to treat HIV infection (British HIV Association, 2008).
Transmissible spongiform encephalopathies
Transmissible spongiform encephalopathies (TSEs) are fatal degenerative brain diseases that occur in humans and several animal species (Goldfarb and Brown, 1995). They are caused by altered forms of naturally occurring prion proteins (PrP) that are normally present in human and animal brain tissue. In TSEs, these altered prion protein forms (PrPSc) accumulate in the brain and subsequently are responsible for the characteristic features of TSEs. These altered prion proteins are extremely resistant to inactivation by standard thermal, chemical, and other means used routinely for destroying microorganisms. The best-known human TSE is Creutzfeldt–Jakob disease (CJD). Person-to-person transmission of TSEs through direct contact does not occur. Iatrogenic (clinical treatment-associated) transmission via contaminated medical devices has been documented on rare occasions. In 1995 a new form of TSE was described in the United Kingdom called variant CJD (vCJD) (Hullard d’Aignaux et al., 2001). A link has been established between vCJD and bovine spongiform encephalopathy (BSE) in cattle and vCJD appears to have arisen through the consumption of BSE-infected animal products. There have been several probable cases of vCJD derived from transfusion of whole blood (Llewelyn et al., 2004) and blood products (Chohan et al., 2010). In humans, prion proteins have been detected in tonsilar tissue, but the majority of oral tissues examined were negative, with the exception of the trigeminal ganglia (Head et al., 2003). However, a recent study showed that following intestinal infection using a murine model with a prion agent related to vCJD, infectivity was widely disseminated in tissues of the murine oral cavity, including salivary glands, dental pulp and gingivae at a higher level than previously described, during the preclinical and clinical stages of the disease (Walker et al., 2009; Walker, 2010). The study also provided evidence for the potential transmission of the disease from contaminated instruments by contact with the gingival margin.
No iatrogenic cases of CJD have been linked to dental procedures and there currently is no evidence of TSE infectivity, including vCJD, in dental tissues. Dental procedures can be considered low risk, assuming optimal standards of IPC and instrument decontamination are maintained (Walker, 2010). For patients who do not have a known or suspected TSE, Standard Precautions (see below) are sufficient for dental procedures. However, procedures that are likely to involve contact with neurovascular tissue should, if possible, be scheduled for the end of the treatment session, to allow time for appropriate cleaning and decontamination of instruments. Although there is no evidence of increased risk associated with performing dental procedures on known or suspected TSE patients, the following precautions are recommended:
- Single-use instruments should be used and destroyed following use by incineration.
- Dental handpieces should not be attached to dental chair unit (DCU) waterlines as there is a small possibility that potentially infected clinical material could be drawn into the water supply line due to antiretraction valve failure (see below). If irrigation is required, it should be provided using a disposable syringe.
- A portable suction unit, with disposable reservoir and suction tubes, should be used.
- Patients should rinse their mouth during and following dental treatment into a disposable bowl, rather than the DCU cuspidor.
For the interested reader, more comprehensive overviews on TSEs are provided elsewhere (Hullard d’Aignaux et al., 2001; Taylor, 2004; Bennett et al., 2007; Department of Health, 2007).
Immunisation
Immunisation or vaccination is an essential part of IPC programmes for dental healthcare workers, and a comprehensive immunisation policy should be implemented and monitored for all dental healthcare facilities. It is important to appreciate that vaccinations do not always result in immunity and that there are some diseases for which vaccination does not yet exist (e.g. HCV and HIV). For all dental healthcare personnel, the immunisation policy should incorporate current legislation and the guidelines and recommendations provided by national healthcare authorities. Immunisations should be carried out before the dental healthcare worker is placed at risk, preferably during training and prior to exposure to patient-derived clinical material (e.g. contaminated dental instruments). Immunisation should also be considered for non-clinical staff who, because of their duties, may be placed at risk of acquiring infections (e.g. maintenance, clerical, domestic and cleaning personnel). Routine childhood vaccinations include immunisation against poliomyelitis, tetanus, diphtheria, pertussis (whooping cough), measles, mumps and rubella. Vaccinations against meningococcal group C meningitis and septicaemia and Haemophilus influenzae type B are also available. In addition to these vaccines, the dental healthcare worker should receive HBV vaccination and a follow-up blood test(s) to determine adequate serum antibody titre levels (consult your national guidelines with regard to recommended response levels). Annual immunisation should also be considered for influenza viruses, provided this is consistent with the indications of national regulatory authorities. The predominant influenza virus strain(s) circulating during any particular year is monitored by the World Health Organization (WHO) and they recommend the most appropriate vaccine for annual use (World Health Organization, 2010).
Standard precautions
In 1996, the USA Centers for Disease Control and Prevention (CDC) expanded the concept of Universal Precautions for IPC and changed the term to Standard Precautions. Standard Precautions integrate and expand the elements of Universal Precautions into a standard of care designed to protect healthcare personnel and patients from transmission of microorganisms from blood or any other body fluid, excretion or secretion. Standard Precautions apply to contact with all blood, body fluids, secretions and excretions (except sweat), regardless of whether they contain blood (Kohn et al., 2003) (Figure 8.2).
Figure 8.2 Standard Precautions should be applied to all patients regardless of their known or unknown infection status and medical history.

They are also applied in relation to contact with non-intact skin (i.e. damaged or injured skin) and with mucous membranes. In addition to Standard Precautions, other measures may be necessary to prevent transmission of specific infectious diseases (e.g. tuberculosis, influenza and varicella (cause of chickenpox)) that are transmitted through airborne, droplet or contact (e.g. sneezing, coughing and contact with skin). In reality, patients who are acutely ill with these diseases rarely seek routine dental outpatient care. However, a general understanding of precautions for diseases transmitted by all routes is necessary as patients may not always be aware of their disease status (i.e. they may be asymptomatic) or they may deliberately conceal an existing condition from the healthcare worker. Necessary transmission-based precautions might include treating the patient in isolation, adequate room ventilation, respiratory protection (e.g. staff treating a patient with active tuberculosis should wear facemasks designed to exclude tuberculosis-causing bacteria), or indeed a postponement of non-emergency dental procedures.
A combination of Standard Precautions, personal protective clothing and equipment, surgery design and administrative controls is the best means to minimise occupational exposures. Written policies and procedures to facilitate prompt reporting, evaluation, counselling, treatment and medical follow-up of all occupational exposures should be available to all dental healthcare workers. These policies and procedures should be consistent with international and national guidelines and legislation, and local requirements and should address education and training, post-exposure management and exposure reporting. Dental training programmes based in dental schools and hospitals have the advantage of being able to coordinate with departments that provide personnel health services. However, the majority of dental practices are in settings that do not have direct access to comprehensive on-site health service programmes. In this regard, it would be prudent for the dental team leader(s) or practice manager(s) to ensure that appropriate IPC and referral arrangements are in place before any dental healthcare worker is placed at risk of exposure to infectious or potentially infectious microorganisms. The personal health of each member of the dental team is also important. Under certain circumstances, it may be necessary to exclude a member of the team from undertaking or assisting with invasive procedures (e.g. a dental team member colonised by methicillin-resistant Staphylococcus aureus (MRSA) should not be involved with invasive procedures (e.g. surgical procedures) until the MRSA has been eradicated). Exposure occurs most frequently during percutaneous and needlestick injuries. Other exposure methods are through contact with potentially infectious non-intact skin, other body fluids and mucous membranes.
Hand hygiene
Hand hygiene is one of the primary disease prevention procedures for all healthcare workers (Boyce and Pittet, 2002; Kampf et al., 2009). It assists with reducing the numbers of resident microorganisms, predominantly bacteria, and also eliminates the majority of transient microorganisms that are acquired on a daily basis by handling and touching surfaces, instruments and equipment. Hand washing is a procedure where microorganisms and debris are removed with mechanical action and should be undertaken using a liquid soap preparation that conforms to hand washing efficacy standards and dermatological criteria. A liquid soap preparation containing an antimicrobial agent (e.g. chlorhexidine) is recommended for preoperative surgical procedures. Non-antiseptic soap bars or liquid soap should not be used in the dental clinic as these readily become contaminated with bacteria from skin and contaminated sinks and/or water and can therefore act as reservoirs and disseminators of infection (Hegde et al., 2006; Rabier et al., 2008). Liquid soap should be provided in disposable cartridges as refillable soap containers can become contaminated during the refilling process.
The duration of the hand-washing procedure depends on the procedure that is going to be performed: preoperative surgical hand scrub (2–6 minutes), social or antiseptic hand hygiene (15–30 seconds). A hand-washing technique that includes all surfaces of the hand should be used and these have been well described in the literature (World Health Organization, 2009).
Hands should be thoroughly dried after washing as wet surfaces encourage proliferation of bacteria and fungi. Good-quality disposable paper towels should be used for this purpose, as there is a significant risk of contamination when hands are dried using hand towels or roller towels. Poor-quality paper hand towels can cause abrasion or damage to the skin when used repeatedly. Hands should be dried using a patting rather than a rubbing action. Warm air dryers are not appropriate in the healthcare setting as the drying cycle time is often inadequate and they can be a source of cross-contamination (Boyce and Pittet, 2002).
There is considerable evidence in the literature that overall compliance with hand-washing procedures in healthcare settings is less than ideal (Kuzu et al., 2005; Mathai et al., 2010). There are many reasons for this, including lack of education, knowledge or motivation, perceived lack of need, lack of appropriate role models, general inconvenience, insufficient time between procedures or patients, inadequate or inaccessible wash basins and harsh hand-washing products that may cause contact dermatitis or eczema. There is also the potential for hands to become contaminated from microorganisms resident in hand-washing sinks (Coleman et al., 2010). Therefore, dedicated sinks should be assigned and maintained for hand-washing procedures only, and should be regularly cleaned, disinfected and maintained. Furthermore, hands-free taps are also recommended.
In recent years, decontamination of hands by rubbing with alcohol-based solutions or gels has been advocated for use instead of hand washing when hands are visibly clean. Alcohol is more efficient and faster acting than antiseptic soaps and the use of alcohol-containing hand hygiene products helps to improve overall hand hygiene. Alcohols exhibit wide antimicrobial activity against bacteria (including mycobacteria), viruses and fungi, but poor activity against bacterial spores (World Health Organization, 2009). Alcohol alone has no residual effect and to counteract this, biocides such as chlorhexidine, triclosan or quaternary compounds have been added to some hand hygiene products, which increases the persistence of antimicrobial activity on the skin. Alcohol hand gels have many advantages:
- They have a wide antimicrobial spectrum.
- They act rapidly, they evaporate quickly.
- They spread easily on the skin.
- There is no need for a sink or drying facilities.
- They save time compared to conventional hand washing.
There is also evidence that healthcare workers are more likely to use them than to wash hands with soap and water. However, it is important to ensure there is adequate time to allow the hands to dry prior to putting on gloves. Use of towels or tissue to dry hands after application of alcohol hand gels may lead to recontamination. Alcohol hand gels may cause drying of the skin after repeated use and some products have added emollients to counteract this. Finally, a sticky residue may build up on the hands following several successive hand gel applications and some manufacturers recommend that hands should be washed periodically with soap and water to remove this.
Finger nails, hand and wrist jewellery
All wrist and hand jewellery should be removed prior to performing clinical procedures and before performing hand hygiene or donning gloves. Bacterial densities on hands are higher when rings are worn, particularly those containing stones or ridges. Wrist and hand jewellery interfere with thorough hand washing and make putting on gloves more difficult and increase the likelihood of gloves becoming damaged in the process and should not be worn. Nails, especially the underside, harbour the highest densities of microorganisms so they should be kept short, filed smoothly and free of varnish. Artificial nails (of any kind) increase microbial load and discourage vigorous hand washing and should not be allowed in a healthcare environment.
Aseptic techniques
Medical or clean asepsis is used in an effort to keep patients as free from exposure to microorganisms as possible. It is used to prevent contamination of wounds and other susceptible sites by microorganisms that could cause infection. Surgical or sterile asepsis includes procedures to eliminate microorganisms from an area. This is particularly applicable to theatre suites. Sterile equipment and fluids are used during invasive procedures. Medical or clean asepsis reduces the number of microorganisms present and prevents their spread. In the dental clinic, before patient treatments, it is important to check that instrument packs and kits are sterile and that packs are intact. Before setting up for a procedure in the dental clinic, work surfaces should be clean and dental staff should carry out hand hygiene and wear the appropriate protective clothing and equipment (see below). Staff should not lean or reach over the set-up area. Sterile packs should be opened only from the corners by peeling back and tipping the instruments gently on to the centre of the pre-prepared set-up area or sterile field. Air movement near the set-up should be minimised. Do not allow others to use your treatment area as a passageway.
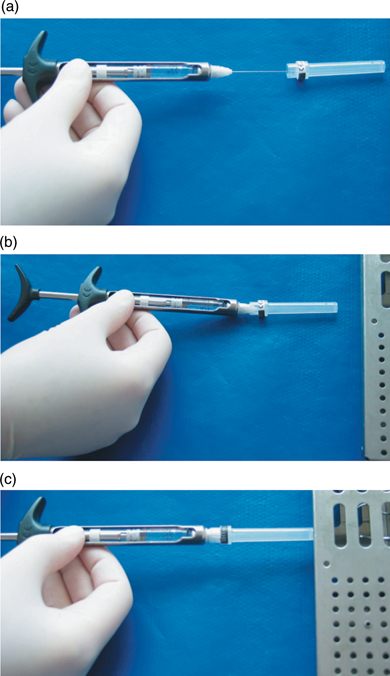
In dentistry, it is necessary to recap local anaesthetic dental needles, as local anaesthetic may have to be readministered during the treatment session. This should be carried out using a single-handed bayonet or scoop technique (Figure 8.3). Alternatively, there are some commercially available devices that hold the needle cap firmly in position. The needle can then be recapped by inserting the needle into the prepositioned cap.
Figure 8.3 Recapping of local anaesthetic dental needles using a single-handed bayonet or scoop technique. (a) & (b) Place the needle cap on the work-top and ‘fish’ it up with the needle when you are ready to recap. Make sure you keep your free hand out of the way of the needle. (c) Push the recapped needle against a firm flat surface to secure the needle cap in position.
Protective clothing
Protecting dental healthcare personnel from potential exposure to microorganisms requires a combination of controls, one of which is the use of personal protective equipment (PPE) (Kohn et al., 2003). The wearing of PPE helps to prevent contact with infectious microorganisms or body fluid by creating a barrier between the worker and the infectious material. National health and safety authorities require workers, including healthcare professionals, to wear PPE when there is a risk of exposure to potentially infectious diseases. PPE should never be worn outside the clinical environment.
Gloves
Gloves protect the dental healthcare worker’s hands from exposure to microorganisms while performing dental procedures. However, they do not protect against percutaneous injuries. A variety of disposable gloves are available for this purpose, the majority of which are made of latex, vinyl or nitrile. Non-sterile, ambidextrous, disposable, latex examination gloves are the most frequently used type of glove in modern dental surgeries. The use of powdered gloves has been discontinued in many healthcare institutions to reduce exposure to latex powder that can cause allergy (Korniewicz et al., 2005). If latex gloves are used, they should be powder-free and low protein gloves (Health and Safety Executive, 2008). Sterile surgical gloves are worn for surgical procedures. Auxiliary dental staff frequently wear disposable latex gloves when cleaning environmental surfaces in the dental clinic; however, they are not suitable or intended for this purpose. Reusable heavy-duty gloves made of latex or nitrile are more appropriate and individual pairs should be provided for each worker concerned and not used by many different individuals.
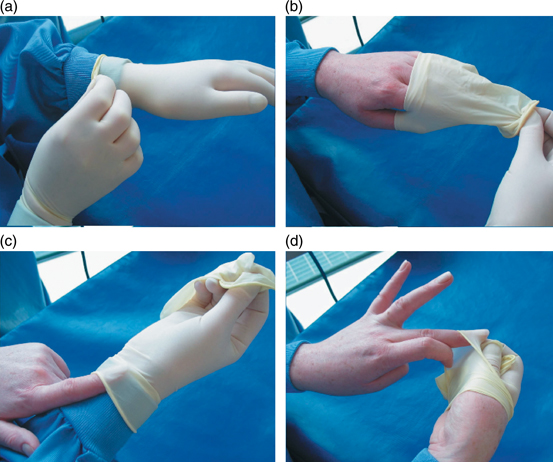
It is important to appreciate that gloves only protect you provided you do not touch environmental surfaces, face or hair with contaminated gloves. Care should be taken to ensure not to touch and thus cross-contaminate items such as pens, case notes, X-ray films, telephones, computers (screen, keyboard and mouse, etc.) with contaminated gloves. Gloves should be changed after each patient and should never be washed or reused. Gloves should also be changed if they become heavily soiled, even if being used during treatment of the same patient, or torn during use. Gloves should also be worn when handling or cleaning items or surfaces contaminated with body fluids (e.g. used clinical gowns and the DCU spittoon). Hand hygiene procedures should be undertaken before putting on and after removal of gloves. Care should be taken to limit self-contamination when removing gloves. Remove gloves by grasping the outside edge of the glove closest to the wrist; peel the glove away from the hand and in so doing, the glove will be turned inside out. Then slide an ungloved finger under the cuff of the remaining glove and peel it off from inside (Figure 8.4) (World Health Organization, 2009). Discard used gloves into a contaminated waste bin.
Figure 8.4 The correct way of removing contaminated disposable gloves used during dental procedures. (a) Remove gloves by grasping the outside edge of the glove closest to the wrist. (b) Peel the glove away from the hand and in so doing, the glove will be turned inside out. (c) & (d) Then slide an ungloved finger under the cuff of the remaining glove and peel off from inside.
Gowns
Clean gowns should be worn to prevent cross-contamination of the clothes of dental healthcare staff during dental procedures. It is important to emphasise that uniforms (e.g. dental nurses’ or dental hygienists’ uniforms, etc.) are not protective clothing and protective gowns should be worn over uniforms during dental procedures. There are many different views regarding whether gowns should have long or short sleeves; however, long-sleeved gowns protect the forearms from contamination and are recommended. Gowns should protect the torso and be long enough to cover the knees of operators while in the seated position. Gowns should also be fluid resistant and, in the case of reusable gowns, be able to withstand high-temperature washing with a good-quality detergent. Gowns should be changed if they become visibly soiled. Gowns should be removed by loosening the ties or snap fasteners at the back of the gown, which should be uncontaminated, and then peeling the gown away from the neck and shoulders. During this process the contaminated outside part of the gown should be facing away from the wearer. The gown may then be rolled into a bundle and placed in a designated laundry bin in the case of reusable gowns, or a designated contaminated waste bin as appropriate. Gowns are the first item of PPE to be put on and the last to be removed before leaving the patient treatment area.
Facemasks
Standard facemasks are used to protect the mucous membranes of the mouth and nose from spray, splashes, spatter and aerosols. However, they only offer limited protection against respiratory pathogens like influenza virus. They are secured in place with either string ties at the back of the head and base of the neck or by adjustable elastic tapes. The mask should be adjusted to fit snugly against the face and nose so that there are no gaps. The mask should not be touched during procedures to prevent contamination from gloves. Face shields may also be worn and should protect the forehead, extend below the chin and also wrap around the sides of the face. Masks should be changed after each patient or if visibly soiled or wet. Facemasks should not be worn around the neck as a necklace as this serves no useful purpose. Masks should be removed by untying the strings from the bottom first and then the top and then discarding into a designated waste bin.
Protective glasses
Protective glasses or goggles should be worn during dental procedures by both dental healthcare workers and patients to prevent physical injury and transmission of microorganisms to the eyes. Eyes are particularly vulnerable to injury by high-velocity particles/debris generated during use of high-speed handpieces and scalers. Prescription />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


