Tissue Engineering
Tissue engineering is a term coined at a meeting sponsored by the National Institutes of Health in 1987. This discipline is a rapidly developing multidisciplinary branch of science that combines many of the basic principles of biology, medicine, and engineering. The primary goal of tissue engineering is the restoration, maintenance, or enhancement of tissue and organ function.
In addition to having therapeutic applications, in which a particular organ is custom grown to replace a failing or missing body part, tissue engineering has diagnostic applications in which tissues are fabricated in vitro and used for in vitro biocompatibility testing of compounds. Examples include application in drug metabolism and uptake, toxicity, or pathogenicity. Tissue engineering research, therefore, translates fundamental knowledge in physics, chemistry, and biology into materials, devices, and strategies. It also integrates biomaterials, cell biology, and stem cell research; engineering characteristics of three-dimensional structures and mass transport issues; biomechanical characteristics of native and replacement tissues, biomolecules and growth factors; and bioinformatics to support gene/protein expression and analysis.
Tissue engineering as a discipline grew out of the pressing need for replacement tissues and organs. During 2010, in the United States alone, over 28,600 solid organs (heart, lung, intestine, kidney, pancreas, and liver) were transplanted. More than three quarters of those organs came from deceased donors. Meanwhile, during this same period, 56,437 new potential recipients were added to the waiting list and nearly 7000 patients died while waiting for a transplant (Box 16-1). Clearly, the demand for organs greatly exceeds the supply. Enthusiasm for tissue engineering comes from the promise of making transplants easier and more common. In dentistry, the disciplines of periodontology and oral and maxillofacial surgery often use bone tissue transplant materials to repair defects.
Today, there are four primary classes of tissue/organ transplants: autograft, allograft, xenograft, and alloplast (Box 16-2).
Autograft
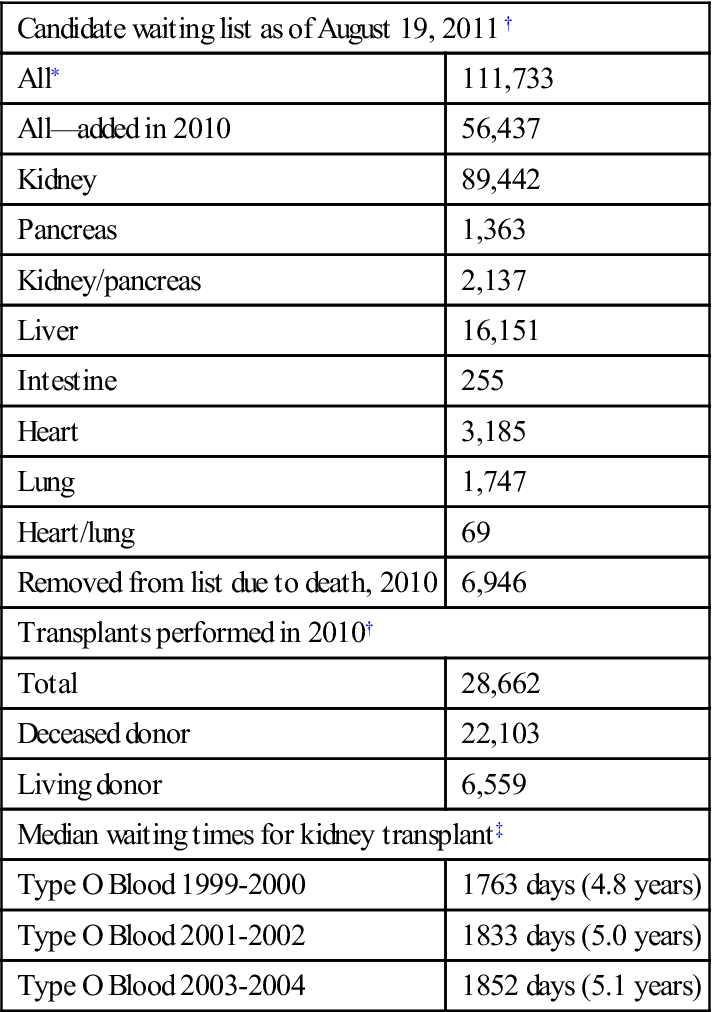
An autograft is a tissue or organ that is transferred from one location to another within a single individual (Figure 16-1). It is common to transplant tissues such as hair, blood, and even limited amounts of skin and bone. These tissues regenerate to some extent, repairing the void left after their removal. This method of transplantation avoids immunologic complications and is considered the “gold standard” for success.
Allograft
Allografts are tissues or organs that are transplanted from one individual to another within the same species (Figure 16-2). Routinely, tissues and organs are removed from deceased individuals (as well as living donors) and transferred to a different individual. Blood, bone, skin, corneas, ligaments, and tendons are collected in banks and frozen, to be used in future surgical procedures.
Xenograft
The Center for Biologics Evaluation and Research (CBER) is the Food and Drug Administration (FDA) branch that oversees and regulates human tissue transplants. They define xenotransplants as “transplantation, implantation, or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source or (b) human body fluids, cells, tissues, or organs that have had ex vivo contact with live nonhuman animal cells, tissues, or organs.” This therapeutic regimen has been used experimentally to treat neurodegenerative disorders, liver failure, and diabetes, when compatible human materials are not widely available.
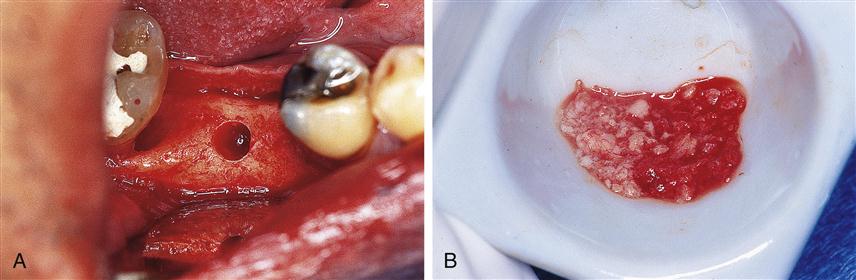
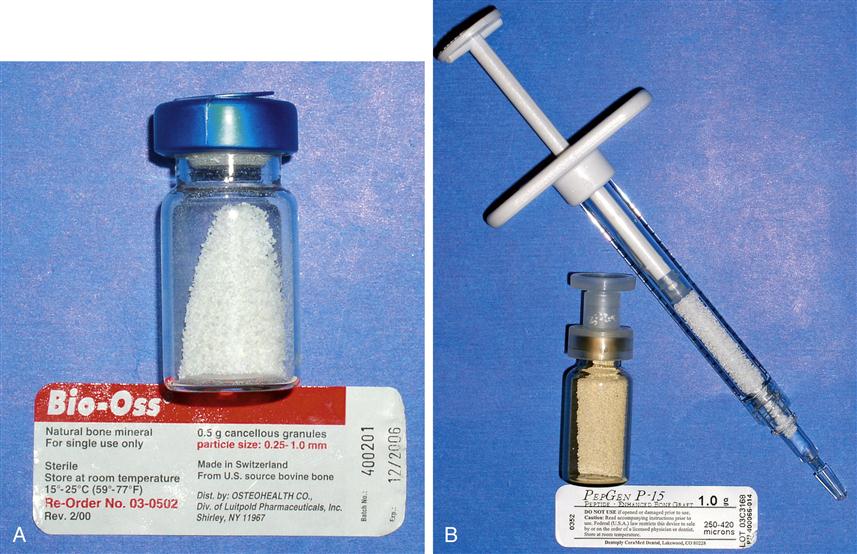
Xenografts are now common in dentistry. Two examples are BioOss (a product derived from cow bone) and BioCoral (a corraline product) that are used to augment defects in the maxilla and mandible (Figure 16-3).
Alloplasts
Alloplasts are the newest type of grafting procedure materials. These grafts are fabricated completely from synthetic materials, making them quite different from the other three types of grafts, because no living component is used. Alloplasts, such as dental implants, are becoming increasingly common in dentistry. Dental implants fabricated from metals and ceramics are considered routine restorative treatment in many countries. These materials integrate with bone and help restore function for the patient, with excellent long-term success.
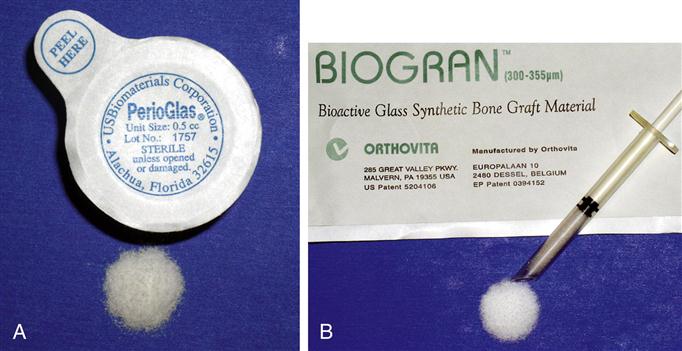
Bone grafting alloplasts are also common (Figure 16-4). Autograft bone placed in the reconstruction of craniofacial structures can be augmented with ceramic and bioactive glasses. These alloplasts are available in nearly unlimited quantity with no adverse immunological reaction. An important benefit is that they do not pose the risk of transmitting disease from one individual to another.
Strategies for Tissue Engineering
Tissue engineering began with the concept of using biomaterials and cells to assist the body in healing itself. As the discipline matured, its goal shifted to developing logical strategies for optimizing new tissue formation through the judicious selection of conditions that will enhance the performance of tissue progenitors in a graft site, ultimately encouraging the production of a desired tissue or organ. Several strategies are now available for developing new organs and tissues (Box 16-3).
Injection of Cells
With the cell injection method, disaggregated cells at an undifferentiated stage of development are injected into the recipient. Considerable research had been conducted into the use of this method to combat systemic diseases such as Alzheimer’s and Parkinson’s disease, as well as juvenile-onset diabetes and multiple sclerosis. It also holds potential for treating damaged nerve and muscle sites. Commonly referred to as stem cells, the injected cells are more appropriately termed undifferentiated or progenitor cells (see later discussion under Stem Cells). These cells are capable of forming new tissue with one or more phenotypes. As a therapeutic regimen, the cells are injected into the vicinity of the site in which they are intended to propagate, and they migrate to the area of injury and begin to replicate and replace the lost tissue, or produce a desired compound such as insulin (Figure 16-5). This strategy has already been successful in regenerating small areas of cartilage in temporomandibular joints.
Guided Tissue Regeneration
Guided tissue regeneration (GTR) is a surgical procedure for regenerating tissue by enhancing the opportunity for one cell type to populate an area while providing contact guidance to the developing cells. The desired cell types can then populate an area without competition because unwanted cell types are excluded. In the laboratory, the method has been successful in creating a biodegradable polymer conduit through which nerve cell regeneration and reconnection can occur. GTR is commonly used in periodontal treatment to regenerate lost periodontal tissues such as the bone, periodontal ligament, and connective tissue attachment that support the teeth. The procedure involves placement of a membrane under the mucosa and over the residual bone (Figure 16-6). The barrier helps to exclude the faster-growing epithelium and gingival connective tissues during the postsurgical healing phase, allowing the slower-growing periodontal ligament and bone cells to migrate into the protected areas.
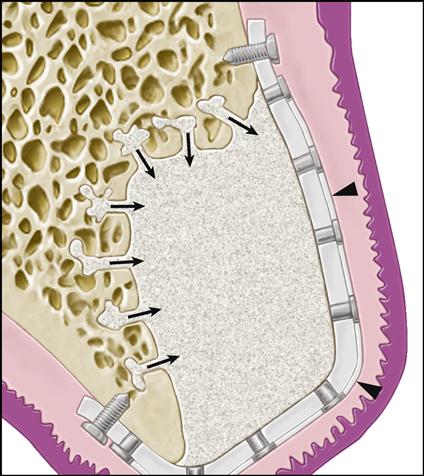
GBR is being used to isolate the site being regenerated from the overlying tissues by means of a titanium-reinforced membrane barrier (arrowheads). The newly forming bone may be augmented by the addition of particulate grafting material. The barrier is securely attached to the margins of the defect to prevent displacement during healing. Notice that decortication of the underlying bone bed allows invasion of the site by osteogenic precursor cells from the blood stream and surrounding bone.
Cell Induction
In the last decade there has been explosive growth in understanding the role of cytokines, developmental proteins, and growth factors in molecular biology. Many of these growth factors are available as recombinant human lyophilized proteins. There have been tremendous advances in administering various mixtures of these growth factors directly to tissues to force them down a particular differentiation lineage. Often these growth and differentiation factors can be simply injected into the site. This technique, as a tissue-engineering regimen, targets local connective tissue progenitors already present in the region where new tissues are desired and induces those cells to generate the desired tissue. Some of the injected proteins may serve as mitogens in recruiting cells to migrate into the area, where other growth factors cause them to differentiate. Alternatively, they can be delivered by a substrate that releases them over time.
Gene therapy can be used with cell induction methods. In this application, a gene sequence that encodes for production of a particular growth factor or therapeutic compound is inserted wholly into the genome of the recipient cell. Insertion is achieved by a carrier, called a vector, to deliver the therapeutic gene to the patient’s target cells. The most common vector is a genetically altered virus that has been modified to carry human DNA. Because viruses have evolved a way of encapsulating and delivering their genes to human cells, they can be manipulated to insert these therapeutic genes into the recipient cells. So, vectors are injected into a site along with an initial bolus of the therapeutic compound, and the vectors then upload their genes into resident cells. After incorporating this DNA into their genome, the newly transfected cells begin to replicate the desired growth factor endogenously. This method promotes continuous protein production at the site long after the initially injected growth factors diffuse from their target tissues or degrade enzymatically.
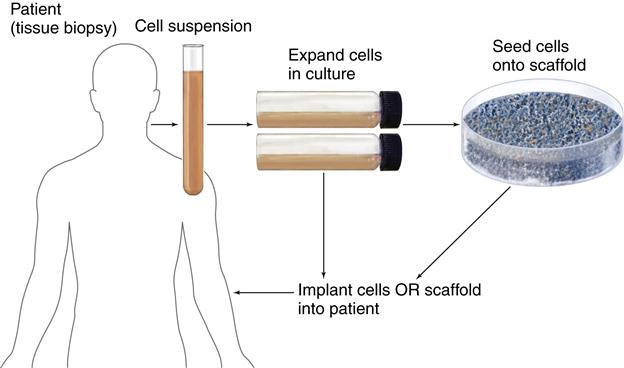
Cells Within Scaffold Matrices
Three-dimensional porous scaffolds can be used with cells to provide many of the advantages of the methods described above. These preformed scaffolds are usually made of bioresorbable materials. The scaffolds promote new tissue formation by providing a surface and void volume that encourages attachment, migration, proliferation, and the desired differentiation of connective tissue progenitors throughout the region where new tissue is required. Typically, the scaffold is seeded with progenitor cells that are allowed to attach and proliferate in vitro. The cell constructs are often grown in a nutrient media supplemented with growth factors necessary for cell and tissue development. During the growth phase, a static or dyn/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses