Materials for Adhesion and Luting
Cementation is one of the final steps in the sequence of clinical procedures for indirect restorations. There are two objectives for the cementation, or luting, procedure: to help retain the restoration in place and to maintain the integrity of the remaining tooth structure. Retention is achieved by friction (or micromechanical interlocking), by an adhesive joint consisting of the prepared tooth, the cement, and the restoration, or a combination of both mechanisms. An effective interfacial seal depends on the ability of the cement to fill the irregularities between the tooth and the restoration and to resist the action of the oral environment, short and long term. Adhesion is also important in this context, because a strong bond between the luting agent and the dental substrates may help prevent bacteria from colonizing the interface and minimizing the transit of fluids that may cause dentin hypersensitivity.
This chapter presents the basic aspects of the application of adhesion science to dentistry and describes the composition, properties, manipulation, and indications for use of acid-base and resin-based cements. Acid-base cements are easy to use and, when correctly indicated, provide good long-term clinical service. Some release fluoride and bond to tooth structures. Resin cements have a chemistry based on resin composites. They show high bond strengths to tooth structures. Some products also contain monomers or are compatible with primers that enable bonding to metal alloys and ceramics. In general, resin cements have better mechanical properties than acid-base cements, but the cementation process is more technique sensitive.
The fundamental technologies and chemistries used to formulate the various types of adhesives and luting cements are derived from their corresponding restorative materials. However, in most cases modifications have been made to create formulations suitable for a particular clinical application in terms of viscosity and handling characteristics.
Different clinical situations require different luting agents and no one material is indicated for every case. Therefore, it is important to differentiate luting cements based on their mechanical properties and overall characteristics to identify the best options available for each clinical situation.
Principles of Adhesion
The creation of a strong, durable, and bonded interface with enamel or dentin provides important benefits. It significantly protects the restoration’s interface against penetration of bacteria that may cause secondary caries. It reduces the need for retentive areas in the preparation that would require removal of sound tooth structure. In some cases, bonding may help strengthen the remaining tooth structure. The development of adhesive luting techniques also broadened the application of materials such as low-strength ceramics and indirect composites for crowns, inlays, and onlays.
The term adhesion refers to the establishment of molecular interactions between a substrate (adherend) and an adhesive brought into close contact, creating an adhesive joint (Figure 13-1). Cohesion is used to describe the interaction of similar atoms and molecules within a material, involving primary (i.e., covalent or ionic) or strong secondary forces (i.e., hydrogen bonding).
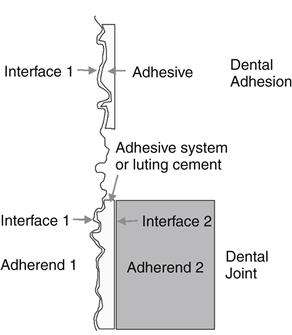
Most dental joints involve at least one adhesive, two substrates, and two interfaces.
In dentistry, true chemical bonding between the tooth structure and restorative or luting materials is very difficult to achieve, because of the complex composition of some substrates such as dentin, the presence of contaminants, and the presence of water. Zinc polycarboxylate, glass ionomer, resin-modified glass ionomer, and self-adhesive resin cements are examples of dental materials capable of establishing chemical interaction with hydroxyapatite. However, in daily practice, adhesion is accomplished by micromechanical interlocking between the adhesive and the substrate. It is important to point out that when two materials are in close contact, physical bonding is always present (e.g., van der Waals dipoles); however, it is weak and does not really contribute significantly to the integrity of the adhesive joint.
A dental sealant attached to enamel is an example of a simple adhesive joint with one interface. Often times, however, adhesive joints involve more than one interface (e.g., tooth/adhesive and adhesive/restorative or luting material), which presents an extra challenge because an adhesive does not necessarily bond equally well to different substrates (see Figure 2-1.
The most basic aspect to be observed in creating any adhesive joint is the cleanliness of the substrate. Saliva, biofilm, and other organic debris are always present on the tooth surface. The walls of a cavity preparation are covered with a smear layer. All of these contaminants reduce the surface energy of the bonding substrate and, consequently, its wettability. Therefore, it is very important for the surface that will contact the adhesive to be thoroughly clean and, in some cases, for the smear layer to be removed by acid etching. Indirect restorations also need to have their internal surface cleaned and free from films that may impede the penetration of the adhesive.
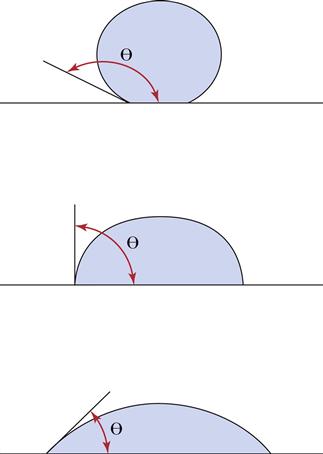
Wettability is the result of molecular interactions between the adhesive and the substrate, as well as the cohesion forces of the adhesive, particularly its surface tension. Liquids tend to form spheres when placed on a surface because that is the shape with the lowest surface area and, therefore, the minimum surface energy (Figure 13-2). Wetting is usually evaluated by the contact angle (θ), that is, the internal angle between the liquid and the substrate. Generally, small contact angles are achieved when a low surface tension liquid is placed on a high-energy surface substrate. Contact angles less than 90 degrees indicate a favorable wetting of the surface. Ideal wetting occurs when the liquid spreads over the surface with θ ≈ 0 degrees. Surface roughness increases the wettability of the surface by liquids.
Viscosity influences the contact of the adhesive with the substrate. It should be low enough to allow the adhesive to flow readily and penetrate into the details of the substrate surface, without leaving porosities at the interface. Finally, the adhesive must set sufficiently to create strong interlocks with the substrate microstructure to achieve micromechanical retention.
Adhesive Systems
Classification and Basic Components
Adhesive systems can rely on different approaches to obtain a strong and durable bond to dentin and enamel. They are classified according to the etching strategy as etch-and-rinse or self-etch. Etch-and-rinse (also referred to as total-etch) systems can be presented as three-step systems, that is, etching, priming, and bonding in separate application steps. Alternatively, two-step systems present primer and bonding resin mixed in a single component. Etching uses 30% to 40% phosphoric acid gels to demineralize the tooth structure. Acid etchants are also called conditioners to disguise the fact that most are relatively strong acids (pH less than 1.0). Originally, etching solutions were free-flowing liquids and were difficult to control during placement. Gel etchants were developed by adding small amounts of microfiller or cellulose thickening agents. These gels flow under slight pressure but do not flow under their own weight.
Primers are hydrophilic monomers, oligomers, or polymers, usually carried in a solvent. The solvents used in primers are acetone, ethanol-water, or primarily water. In some primers, the solvent levels can be as high as 90%. Therefore, primers have different evaporation rates, drying patterns, and penetration characteristics, all of which can influence the resulting bond strength. Dimethacrylate oligomers and lower-molecular-weight monomers can be added to the primer in two-step etch-and-rinse systems, or presented as a separate step in three-step systems or in self-etch two-step systems.
Self-etch systems contain ester monomers with grafted carboxylic or phosphate acid groups dissolved in water. According to their aggressiveness, these systems can be divided into strong (pH of 1 or less), moderate (pH between 1 and 2), or mild (pH of 2 or greater). They can be presented as two-step systems, with a hydrophobic bonding resin in a separate bottle (also known as self-etching primers) or single component systems (all-in-one systems).
Most bonding agents are light-cured and contain an activator such as camphorquinone and an organic amine. Dual-cured bonding agents include a catalyst to promote self-curing. Although most bonding agents are unfilled, some products contain nanofillers and submicron glasses ranging from 0.5% to 40% by weight. Fillers are described in more detail in Chapter 9. Filled bonding agents may be easier to place on the tooth and may produce higher in vitro bond strengths. Bonding agents may contain fluoride, antimicrobial ingredients, or desensitizers, such as glutaraldehyde. The effectiveness of fluoride and antimicrobial release from a bonding agent has not been demonstrated.
In Vitro Evaluation of Bond Performance
Laboratory tests have been extensively used to compare the bond performance of adhesive systems. Though clinical relevance of in vitro evaluations is questionable, they certainly represent a valuable “screening” tool. Also, different than clinical studies, laboratory evaluations allow isolation of specific variables that may interfere with bond performance, for example, substrate conditions, contaminants, application procedures, and thermal and mechanical cycling.
Bond strength tests are, by far, the most popular among in vitro methods. ISO/TS11405 (2003) describes test protocols for both shear and tensile bond strength tests (Figure 13-3). Both tests use relatively large bonding areas (3-6 mm in diameter, 7-28 mm2). Nominal (average) bond strength is calculated by dividing the failure load by the specimen cross-sectional area. The high incidence of cohesive failures of the substrate observed with these tests prompted the development of micro bond strength tests (Figure 13-4), using specimens with much smaller bonding areas (1 mm2). The main limitation of bond strength tests, despite their great popularity, is that results from different studies cannot be directly compared because of the lack of standardization among research groups. Also, because of the heterogeneous stress distribution along the bonded interface, the nominal bond strength value is far from representative of the true stress that initiated debonding.
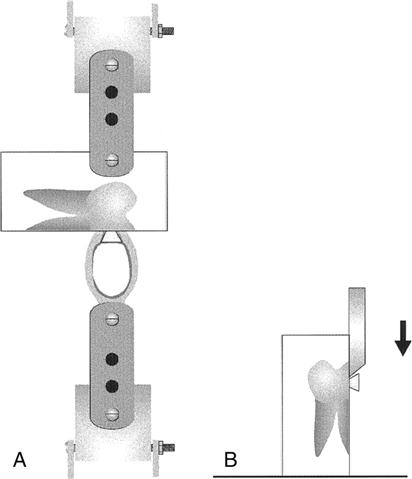
A, Diagram of the tensile test apparatus; B, diagram of the shear test apparatus. (From Cardoso PEC, Braga RR, Carrilho MRO: Dent. Mater. 14, 394-398, 1998.)
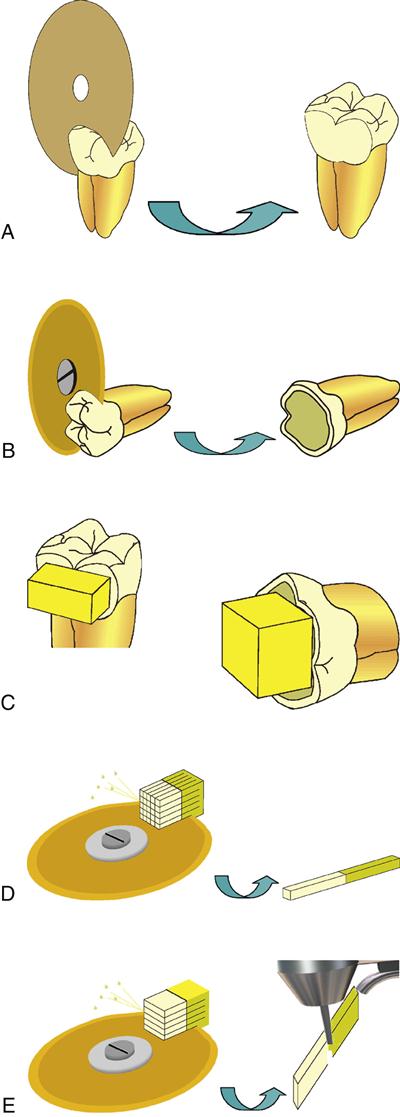
A, Enamel specimens preparation involved the removal of a portion of superficial tissue without exposing the underlying dentin. B, Tooth prepared for dentin test: the occlusal third was removed with a diamond disc, creating a flat surface.C, Resin build-up over the enamel and the dentin surface. D, Cutting of the tooth along the X– and Y-axis and the resulting sticks. E, Procedure for the preparation of hourglass-shaped specimens: the bonded tooth is sectioned in multiple slabs. On each slab the narrowest cross section is created at the interface by trimming with a bur. (From Goracci C, Sadek FT, Monticelli F, et al: Dent. Mater. 20, 643-654, 2004.)
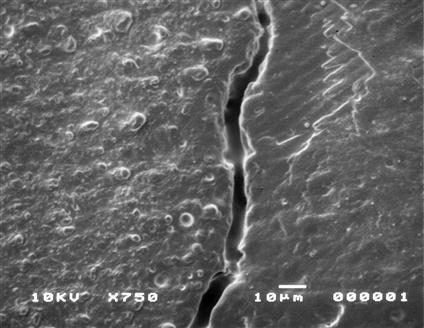
The quality of the marginal seal obtained with adhesive systems can be estimated by different methods. Microleakage tests use the immersion of a restored tooth in a tracer solution (e.g., methylene blue or silver nitrate). The tooth is sectioned and the extent of dye penetration is evaluated, either qualitatively (using scores) or quantitatively. Interfacial gaps can be measured under a scanning electron microscope (SEM). Because processing of the real specimen for SEM viewing is critical and more gaps can be unintentionally created, replicas of the bonded interface in epoxy resin are preferred. The term nanoleakage applies to a method in which specimens previously immersed in silver nitrate are observed under a transmission electron microscope (TEM). The presence of silver deposits demonstrates the presence of gaps and voids at the bonded interface (Figure 13-5).
Other in vitro methods for evaluating the performance of bonding systems are fracture toughness tests that quantify the critical stress level responsible for initiating debonding, and fatigue testing in which the cyclic fatigue resistance after a predetermined number of loading cycles (usually 105 cycles) is calculated.
Biocompatibility
Solvents and monomers in bonding agents are typically skin irritants. For example, 2-hydroxyethylmethacrylate (HEMA) may produce local and systemic reactions in dentists and dental assistants sufficient to preclude their further use in the dental office. It is critical that dental personnel protect themselves from recurring exposure. Protective techniques include wearing gloves, immediately replacing contaminated gloves, using high-speed suction, keeping all bottles tightly closed or using unit-dose systems, and disposing of materials in such a way that the monomers cannot evaporate into the office air. Even with double gloves, contact with aggressive solvents and monomers will produce actual skin contact in a few minutes. All reasonable precautions should be followed, and if unwanted contact occurs, affected areas should be flushed immediately with copious amounts of water and soap. Once the materials are polymerized, there is very little risk of side effects. Although patients should be protected during bonding operations, properly polymerized materials have not been shown to be hazardous to the patient.
Clinical Performance
American Dental Association (ADA) guidelines require adhesives to be tested in restorations for nonretentive class 5 lesions. The lesions, which may be saucer- or notch-shaped, have enamel along the coronal margin and dentin along the apical margin. The success of a bonding agent is evaluated indirectly by examining the performance of the restorations for (1) postoperative sensitivity, (2) interfacial staining, (3) secondary caries, and (4) retention or fracture followed for 18 months. These clinical trials test short-term retention and initial sealing.
Most commercial adhesive systems are successful in clinical trials. However, these clinical trials generally combine enamel and dentin bonding. There is no acceptable clinical regimen for critically testing only dentin bonding in nonretentive preparations. Because clinical trials are usually highly controlled, they are often not predictive of routine clinical use in general practice. Longevity of the bond in general practice may be only 40% of that achieved in clinical trials. Long-term clinical performance of bonding systems for a wide range of materials has not yet been reported.
Sites of failure for most bonded restorations occur along cervical margins where the bonding is primarily to dentin (Figure 13-6). Studies of bonded composites in class 2 restorations have shown that 95% of all secondary caries associated with the composite restoration is in the interproximal area. These margins are the most difficult to seal during placement of the restoration because they are typically bonded to dentin and cementum rather than enamel, and are hard to access with a light guide for adequate polymerization.
The three-step etch-and-rinse systems remain the “gold standard” for adhesive systems, both in laboratory and clinical evaluations.
Enamel Bonding
Bonding to enamel occurs by micromechanical retention after acid etching is used to preferentially dissolve hydroxyapatite crystals in the enamel outer surface (Figure 13-7). Fluid adhesive constituents penetrate into the newly produced surface irregularities and become locked into place after polymerization of the adhesive.
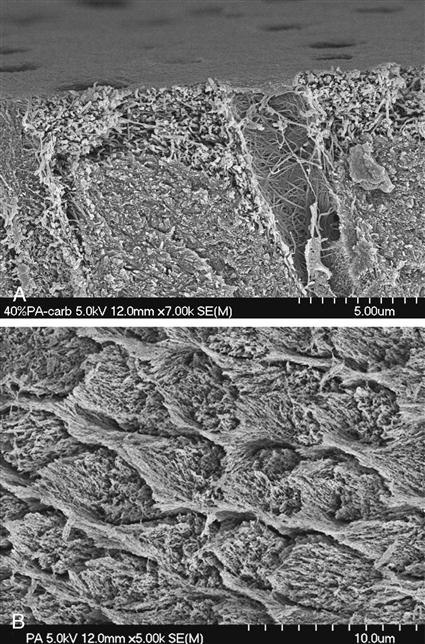
A, Field emission SEM of dentin etched with 40% phosphoric acid for 15 seconds. Note the collagen fibers deprived from hydroxyapatite crystals as a result of acid demineralization. The more intense decalcification around the peritubular area may be a result of both the high mineral content of the peritubular region and the easier penetration of the acid through the tubular lumen. B, Enamel etched with 38% phosphoric acid (Pulpdent) for 15 seconds. (From Perdigão J: Dent. Clin. N. Am. 51, 333-357, 2007.)
Gel etchants (typically phosphoric acid) are dispensed from a syringe onto tooth surfaces to be etched. Etching times for enamel vary depending on the type and quality of enamel. Generally, a 15-second etch with 30% to 40% phosphoric acid is sufficient to reach the characteristic clinical endpoint of a frosty enamel appearance. Deciduous unground enamel generally contains some prismless enamel that has not yet worn away and requires longer etching times (20-30 seconds) to create a retentive pattern. Enamel may have been rendered more insoluble as a result of fluorosis. In those cases, extended etching times (15-30 seconds) are required to ensure that sufficient micromechanical bonding can occur. The only caution is that dentin should be protected from exposure to acid because fluorotic dentin is more susceptible to acid than regular dentin.
After the intended etching time, the acid gel is rinsed away and the tooth structure is dried to receive the bonding resin. If a hydrophilic primer or a two-step etch-and-rinse system is used, the surface can be left moist for the next stage of bonding. Then, primer can be flowed onto the surface to penetrate into the available surface irregularities. After curing, primer and adhesive produce resin macrotags by penetrating the space surrounding the enamel prisms. Microtags form where adhesive flows into the etched prisms involving individual hydroxyapatite crystals. Microtags are much more numerous and contribute to most of the micromechanical retention.
Strong self-etch adhesives produce a similar pattern on enamel as that obtained with phosphoric acid. Mild self-etch systems present lower bond strength to enamel compared to etch-and-rinse systems, probably because of a shallower etching pattern.
Dentin Bonding
The high water content in dentin represents an extra challenge for the establishment of an interdiffusion zone. To manage this problem, primers have hydrophilic components, such as HEMA, that wet dentin and penetrate its structure. In etch-and-rinse systems, etching with phosphoric acid removes the mineral content, creating microporosities within the collagen network. Once the hydroxyapatite component of the outer layer of dentin is removed, dentin contains about 50% unfilled space and about 20% remaining water. After acid is rinsed, drying of dentin must be done cautiously. Even a short air blast from an air-water spray can inadvertently dehydrate the outer surface and cause the remaining collagen scaffold to collapse onto itself. Once this happens, the collagen mesh readily excludes the penetration of primer and bonding will fail. However, excess moisture tends to dilute the primer and interfere with resin interpenetration. The ideal dentin moisture level varies according to the solvent present in the adhesive. With that respect, self-etch systems have the enormous advantage of eliminating this rather subjective step of the bonding procedure.
The infiltration of resin within the collagen scaffold is termed hybridization (Figure 13-8). The result of this diffusion process is called resin-interpenetration zone or resin-interdiffusion zone or simply hybrid layer. Concurrent with hybrid layer formation is the penetration of primer into the fluid-filled dentinal tubules. This generates quite large resin tags. However, these appear to be of little value to overall bonding. This material is generally undercured and behaves as soft flexible tags. If dentin is dehydrated before priming and bonding, these resin tags are more likely to be quite extensive.
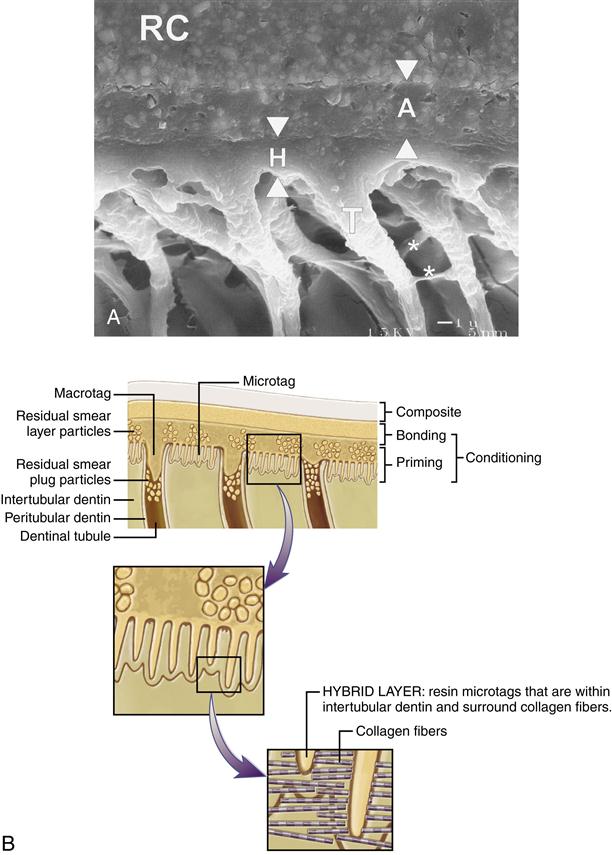
A, Scanning electron micrograph of interface bonded with a dental adhesive (final magnification: ×4000). Note the visible thickness of the adhesive layer (A, arrowheads) beneath the resin composite (RC). The hybrid layer (H, arrowheads) is 2 μm thick. The tubular resin tags (T) show lateral branches (asterisks). B, Schematic showing that etching removes hydroxyapatite crystals within intertubular dentin and along peritubular dentin. Primer penetrates intertubular spaces and fluid-filled tubular spaces. Cured primer forms microtags within intertubular dentin and macrotags within tubules. (A, from Frankenberger R, Perdigão J, Rosa BT, et al: Dent. Mater. 17, 373-380, 2001.)
Primers contain solvents to displace the water and carry the monomers into the microporosities in the collagen network. During application of the primer, most of the solvent evaporates quickly. Thus several layers usually must be applied to ensure a complete impregnation. The rule of thumb is that one should apply as many layers as are necessary to produce a persisting glistening appearance on dentin.
The thickness of a hybrid layer is not a critical requirement for success. Dentin bond strength is probably proportional to the interlocking between resin and collagen, as well as to the “quality” of the hybrid layer, not to its thickness. Effective etching of dentin does not require long times to produce acceptable dentin bond strengths. Usually, 15 seconds is employed. If etching time is too long and the etched zone is too deep, the decalcified dentin may not be fully impregnated. The etched but not impregnated space may reside as a mechanically weak zone and promote nanoleakage. Although this zone has been detected in laboratory experiments, the clinical results of this process have never been demonstrated to be a problem.
After priming the surface, an adhesive is applied and light cured. Surfaces of the cured bonding agents are initially air inhibited and do not immediately react. However, as composite is placed against the surface, the air is displaced and copolymerization occurs.
Self-etch systems have the great advantages of eliminating the risk of incomplete primer/adhesive penetration into the collagen scaffold and also eliminating the subjectivity when determining the amount of moisture on the dentin surface ideal for primer diffusion. With these systems, the smear layer is dissolved and incorporated into the hybrid layer. The bonding mechanism for strong self-etch adhesives is very similar to that of etch-and-rinse systems. Their bond strength, particularly for all-in-one systems, is relatively low, probably because of their high initial acidity and high water content. Mild self-etch systems demineralize dentin only superficially (a few microns) and leave residual hydroxyapatite attached to collagen fibers. Although the main bonding mechanism is the interlocking between collagen fibers and the polymerized resin, monomers such as 4-META (4-methacryloxyethyl-trimellitic anhydride) and 10-MDP (10-methacryloyloxydecyl dihydrogen phosphate) may bond to this residual hydroxyapatite. Also, the presence of hydroxyapatite may help protect the collagen against degradation, which weakens the bonded interface. Mild self-etch systems may present relatively low bond strength values when applied to sclerotic dentin. Another drawback associated with all-in-one systems is that, due to their high water content, they behave as semipermeable membranes, which increases degradation by hydrolysis.
Bonding to Other Substrates
Cast Alloys
Sandblasting with aluminum oxide is the most commonly used method to prepare metal substrates for receiving bonding resins or resin cements. It creates a micro-retentive, high energy surface. Electrolytic etching can be used with base metal alloys, but is not as effective with noble alloys because of its more homogeneous microstructure. Tin-plating can be used to improve the retention of noble alloys to resin cements. Commercial systems using silica-coating at high temperatures or tribochemical application of a silica layer using aluminum oxide modified by silicic acid have also been available for many years. In both cases, a silane solution is applied to the treated metal to create a surface capable of bonding to dimethacrylate-based resins.
Monomers such as 10-MDP and 4-META are used in formulations of resin cements to improve retention of cast alloy restorations. They seem to be more effective with base metal alloys, compared to noble alloys. Metal primers developed for improving the bond strength between alloy and resin cements are also available. However, research results are inconsistent.
Ceramics
Low-strength, silica-based ceramics have been successfully bonded to resin cements by etching the restoration’s inner surface with a hydrofluoric acid solution, followed by the application of a silane primer (Figure 13-9). Different acid concentrations are commercially available, from 2.5% to 10%, in liquid or gel forms, and recommended etching times vary from 1 to 4 minutes. Hydrofluoric acid attacks the glass phase of ceramics, to the point where crystals are removed, leaving a microretentive honeycomb-like, high-energy surface. Silane application improves the wettability of the resin cement on the ceramic surface and establishes covalent bonds with both the ceramic surface (via siloxane bonds, -Si-O-Si-) and the resin cement (by carbon double bond polymerization). Hydrolysis of the silane molecule is necessary to convert the methoxy groups (-OCH3) to silanol (-Si-OH). Silanes are presented in a nonhydrolyzed form (two bottles) or prehydrolyzed (one bottle). In general, prehydrolyzed silanes are less stable, with shorter shelf-life than nonhydrolyzed solutions.
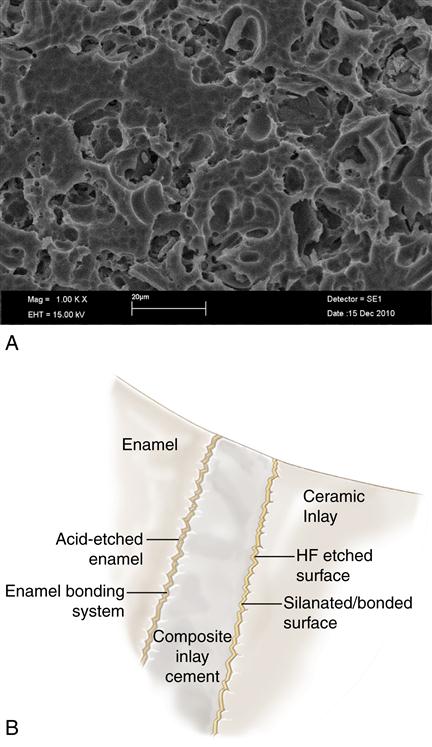
A, Scanning electron micrograph (SEM) of etched porcelain. B, Schematic of materials and interfaces involved in bonding all-ceramic restorations to tooth structure. (Part A From Cesar PF, Yoshimura HN, Miranda Junior WG, et al: Correlations between fracture toughness and leucite content in dental porcelains. J. Dent. 33(9):721-729, 2005.)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses